HBP activation and apoptosis contributed to contractile dysfunction. The heart functional data are consistent with our earlier work and reveal attenuated contractile function without significant alterations to heart rate. Here the 6dP/dt findings implicate the myocardial calcium handling pathway, as diastolic calcium is a key determinant of contractile function and calcium signaling. Since PI treatment decreased and increased myocardial UPS activity and ubiquitination, respectively, this may lead to an accumulation of contractile protein aggregates and impaired cardiac contractility and signaling pathways. For example, protein turnover of AB1010 msds connexin 43, PLB and SERCA-2a are all regulated by the UPS and may explain the higher expression levels found here and before by us. Others have established that altered connexin 43 expression can precede arrhythmias, ventricular fibrillation and incorrect signal propagation in the long-term. Therefore we tentatively suggest that elevated connexin 43 expression in our model may results in detrimental effects on contractile function in the future, especially within the context of HIV-AIDS. We previously identified lower myocardial calcium levels and higher SERCA-2a protein expression with PI treatment, and now report attenuated and elevated calmodulin and pPLB expression levels, respectively. In parallel, we found increased myocardial calcineurin and NFAT3 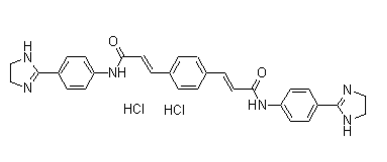 expression levels. Of note, others found that cardiac-specific calcineurin overexpression resulted in enhanced pPLB and SERCA-2a expression and diminished phosphorylation and redistribution of connexin 43. This was associated with depressed contractility and cardiac hypertrophy. Here the authors proposed that connexin 43 may be a downstream target of calcineurin and that attenuated connexin 43 levels may be linked to perturbed gap junction assembly and arrhythmogenesis. We propose that a similar scenario may exist in our model and that greater calcineurin activation is linked to elevated connexin 43 expression that may compromise gap junction function. Increased SERCA-2a, connexin 43 and pPLB expression may occur as a result of lower myocardial UPS and have also been implicated as downstream transcriptional targets of calcineurin. Thus, elevated connexin 43 and pPLB expression may represent an adaptive response by PI-treated hearts to improve calcium handling, which may improve cardiac function. Higher calcineurin activation also leads to increased dephosphorylation and translocation of NFAT3 to the nucleus for activation of downstream targets, e.g. PGC-1a and pro-hypertrophic genes. However, since the calcineurin-NFAT3 pathway did not result in cardiac hypertrophy in our model, we are of the opinion that longer-term activation may eventually result in a hypertrophic response. These findings, however, represent a model of altered cardiac physiology and suggest a potential association with PI-induced molecular alterations to key junction and ionic proteins that may precede the onset of contractile dysfunction. Moreover, the metabolic side-effects elicited by PI treatment in our model �C although at a relatively early stage �C may also affect heart function as a downstream target. Thus we do not imply that the protein expression alterations are directly associated with the altered contractility found in our model. Data linking these phenomena are scarce and therefore makes definitive conclusions difficult. Together these findings indicate that perturbed calcium handling may contribute to the TWS119 PI-mediated contractile dysfunction found in our experimental model in the longer term. However, further studies are required to confirm whether this is indeed the case. Since myocardial PGC-1a was upregulated, this implies that PIs exert initial effects at the mitochondrial level. PGC-1a is a welldescribed transcriptional regulator of mitochondrial biogenesis and we propose that higher expression levels may represent an early compensatory response to energetic stress. In agreement with this notion, NRF-1 and mtTFA expression remained unaltered while we previously identified no changes for myocardial ATP levels and AMPKa expression following 8 weeks of PI administration. It is likely that reduced UPS activity in PI-treated hearts may contribute to the increased PGC1a levels here observed. In support, others established that lower UPS-mediated protein turnover in fibroblasts resulted in PGC-1a stabilization and mitochondrial biogenesis, while it can also be rapidly degraded in the nucleus.
expression levels. Of note, others found that cardiac-specific calcineurin overexpression resulted in enhanced pPLB and SERCA-2a expression and diminished phosphorylation and redistribution of connexin 43. This was associated with depressed contractility and cardiac hypertrophy. Here the authors proposed that connexin 43 may be a downstream target of calcineurin and that attenuated connexin 43 levels may be linked to perturbed gap junction assembly and arrhythmogenesis. We propose that a similar scenario may exist in our model and that greater calcineurin activation is linked to elevated connexin 43 expression that may compromise gap junction function. Increased SERCA-2a, connexin 43 and pPLB expression may occur as a result of lower myocardial UPS and have also been implicated as downstream transcriptional targets of calcineurin. Thus, elevated connexin 43 and pPLB expression may represent an adaptive response by PI-treated hearts to improve calcium handling, which may improve cardiac function. Higher calcineurin activation also leads to increased dephosphorylation and translocation of NFAT3 to the nucleus for activation of downstream targets, e.g. PGC-1a and pro-hypertrophic genes. However, since the calcineurin-NFAT3 pathway did not result in cardiac hypertrophy in our model, we are of the opinion that longer-term activation may eventually result in a hypertrophic response. These findings, however, represent a model of altered cardiac physiology and suggest a potential association with PI-induced molecular alterations to key junction and ionic proteins that may precede the onset of contractile dysfunction. Moreover, the metabolic side-effects elicited by PI treatment in our model �C although at a relatively early stage �C may also affect heart function as a downstream target. Thus we do not imply that the protein expression alterations are directly associated with the altered contractility found in our model. Data linking these phenomena are scarce and therefore makes definitive conclusions difficult. Together these findings indicate that perturbed calcium handling may contribute to the TWS119 PI-mediated contractile dysfunction found in our experimental model in the longer term. However, further studies are required to confirm whether this is indeed the case. Since myocardial PGC-1a was upregulated, this implies that PIs exert initial effects at the mitochondrial level. PGC-1a is a welldescribed transcriptional regulator of mitochondrial biogenesis and we propose that higher expression levels may represent an early compensatory response to energetic stress. In agreement with this notion, NRF-1 and mtTFA expression remained unaltered while we previously identified no changes for myocardial ATP levels and AMPKa expression following 8 weeks of PI administration. It is likely that reduced UPS activity in PI-treated hearts may contribute to the increased PGC1a levels here observed. In support, others established that lower UPS-mediated protein turnover in fibroblasts resulted in PGC-1a stabilization and mitochondrial biogenesis, while it can also be rapidly degraded in the nucleus.
Category: GPCR Compound Library
We measured the electroolfactogram odor-induced field potential at the surface of the olfactory epithelium
The site-specific, bi-directional regulation of tau phosphorylation warrants further studies on evaluation of dose and time dependent effects on OGA inhibition. The EOG was recorded SB431542 ALK inhibitor between a glass microelectrode filled with Ringer that touched the surface of a turbinate and the reference electrode. The micromanipulatorallowed the microelectrode to be withdrawn and repositioned repeatedly to the same location on a turbinate. Electrical signals were amplified by a high-impedance preamplifier. The amplified signal was digitized and visualized on a PC using Igor Pro 4 software. 100-ms pulses of air at 10 psi, odorized by passing through a vial containing 99% isoamyl acetate, were directed at the turbinates using a Picospritzer II. A 100-ms pulse of non-odorized air was applied about 5 s before the odorant. Since the response evoked by non-odorized air was a small fraction of the response to the odorant, the EOG amplitudes were not corrected for this. Our criteria for including the EOGs recorded were that the control EOG was at least 2 mV in amplitude and had a time constant of recovery from the peakof less than 1 s. Successive stimuli were separated by at least 2 min. After locating an area of a turbinate that produced an acceptable EOG, the recording electrode was withdrawn. Ringer was applied to the turbinates, left for about 5 min covering the turbinates, and then wicked away. The electrode was repositioned in WZ4002 contact with the same area and several control EOGs were recorded. After applying and wicking a solution from a 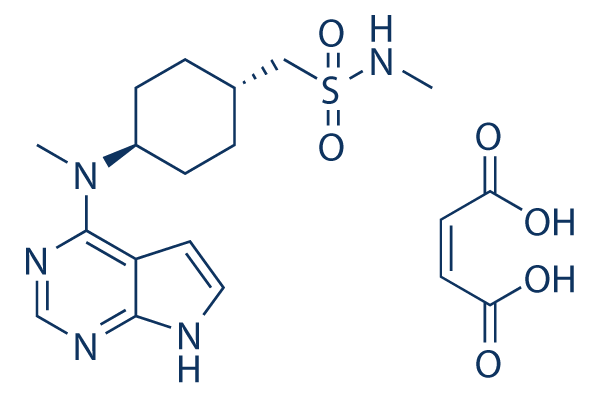 turbinate, the amplitude of the EOG was initially reduced but recovered substantially. Wicking was not able to remove all the added liquid and the change in amplitude was presumably due to the increased thickness of the unstirred layer covering the receptive surface. This layer of liquid could reduce access of the odorant to the tissue and/or could reduce the density of EOG currents. As liquid evaporated or drained off, the EOG amplitude recovered. The amplitude and time constant of the third EOG following a Ringer treatment were usually used for analysis. The electrode was withdrawn again, and the test solution, either Li + -replaced Ringer or Ringer containing 100 mM or 500 mM caloxin, was applied and left for about 5 min covering the turbinates and then wicked. The electrode was repositioned again in contact with the same area and EOGs recorded as above. The recording electrode was withdrawn again and the turbinates were rinsed 3 times with Ringer and then additional Ringer applied for 5 min, wicked, and the EOG recorded again. For a given test solution, only one such experimental series was done on a given turbinate. In some experiments, the second side of the head was also used. The control EOG in every run met the criteria given above for amplitude and time constant. Regardless of the treatment, the recovery of the EOG amplitude from its peak after application of a solution was somewhat variable. One likely source of variability was the exact position of the recording electrode on a turbinate. For example, when recording from a more caudal position on a turbinate, it may have been more difficult to get rid of excess fluid. This might have led to a decrease in the amplitude of the EOG. Based on visual inspection, the rostral portions of turbinates tended to dry out sooner than the caudal portions. To investigate whether PMCA contributes to termination of the odor response, we tested one of the caloxins, a class of selective inhibitors of PMCA. Of the several caloxins available, we selected caloxin 1b1, which has activity against all four PMCA isoforms.
turbinate, the amplitude of the EOG was initially reduced but recovered substantially. Wicking was not able to remove all the added liquid and the change in amplitude was presumably due to the increased thickness of the unstirred layer covering the receptive surface. This layer of liquid could reduce access of the odorant to the tissue and/or could reduce the density of EOG currents. As liquid evaporated or drained off, the EOG amplitude recovered. The amplitude and time constant of the third EOG following a Ringer treatment were usually used for analysis. The electrode was withdrawn again, and the test solution, either Li + -replaced Ringer or Ringer containing 100 mM or 500 mM caloxin, was applied and left for about 5 min covering the turbinates and then wicked. The electrode was repositioned again in contact with the same area and EOGs recorded as above. The recording electrode was withdrawn again and the turbinates were rinsed 3 times with Ringer and then additional Ringer applied for 5 min, wicked, and the EOG recorded again. For a given test solution, only one such experimental series was done on a given turbinate. In some experiments, the second side of the head was also used. The control EOG in every run met the criteria given above for amplitude and time constant. Regardless of the treatment, the recovery of the EOG amplitude from its peak after application of a solution was somewhat variable. One likely source of variability was the exact position of the recording electrode on a turbinate. For example, when recording from a more caudal position on a turbinate, it may have been more difficult to get rid of excess fluid. This might have led to a decrease in the amplitude of the EOG. Based on visual inspection, the rostral portions of turbinates tended to dry out sooner than the caudal portions. To investigate whether PMCA contributes to termination of the odor response, we tested one of the caloxins, a class of selective inhibitors of PMCA. Of the several caloxins available, we selected caloxin 1b1, which has activity against all four PMCA isoforms.
Where geraniin treatment caused apoptosis through up-regulation of the Fas ligand expression
Small library including different phenolic compounds, such as flavonoids, tannins and coumarins. Among the different flavonoids and tannins that were able to bind Hsp90, we focused on the ellagitannin geraniin, the main polyphenolic compound in Geranium thunbergii, a medicinal plant used to treat diarrhea in Japan. Ellagitannins belong to the hydrolyzable tannin group and occur in several food plants, such as raspberries, strawberries, blackberries, pomegranate, almonds, and walnuts. In vitro and in vivo studies have demonstrated various biological activities of this class of compounds, including antioxidant, antiviral, antimutagenic, antimicrobial, and antitumor effects, suggesting that the consumption of ellagitannins might be beneficial to human health. Geraniin is a typical ellagitannin because it is composed entirely of common acyl units, such as galloyl, hexahydroxydiphenoyl, and dehydrohexahy droxydiphenoyl groups. Although variousstudies of geraniin have proved its antioxidant, antitumor, and antivirus properties, its mechanism of action is still poorly characterized. Herein, we report the results of a panel of chemical and biological approaches that demonstrate that geraniin binds to Hsp90a and inhibits its ATPase activity, thus compromising the stability of some oncogenic client proteins. Our results Z-VAD-FMK indicated that geraniin could represent an innovative scaffold for the design of new Hsp90 inhibitors SP600125 interacting with its ATPase domain. Inhibition of HSP90 has received significant attention in cancer research due to its ability to retard or block tumor growth. In this respect, Hsp90 plays a critical role in the maintenance of multiple oncogenic pathways and is required to maintain the folding, stability and functionally active conformation of many aberrant oncoproteins. In healthy cells, Hsp90 is involved in dynamic, low-affinity interactions with a plethora of proteins during folding and maturation; however, in tumor cells, it assists folding of dysregulated oncoproteins and sustains their aberrant activity. Given the diversity of the Hsp90 client proteins involved in critical cellular pathways and processes, inhibition of Hsp90 was predicted to have efficacy in a broad variety of human tumors. However, although several Hsp90 inhibitors have thus far entered into clinical trials, the development of Hsp90 inhibitors has encountered difficulties, including drug solubility and hepatic toxicity. Based on the notion that natural products are compounds pre2optimized by evolution to act against specific biological targets, we performed a structure2based screening of different plant2derived polyphenols to identify new potential Hsp90 inhibitors. By SPR 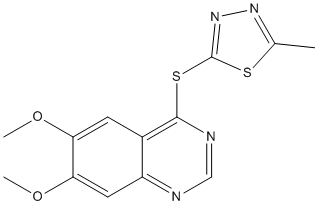 analysis, the tannin geraniin was identified as an efficient ligand of Hsp90a, showing a high affinity for this chaperone, similar to that found for 17-AAG and derivates. The comparison of the HSP90a proteolytic patterns in the presence or absence of geraniin indicated that this compound binds at the Nterminus of the chaperone, as reported for several Hsp90 inhibitors. Through targeting the ATP-binding site of the N-terminal domain, the inhibitors probably prevent Hsp90 from forming a closed N-terminal dimeric state and, consequently, alter the chaperone activity of the molecule. This mechanism was proved for geraniin, which was able to reduce Hsp90 chaperone activity more efficiently than 17-AAG. The impaired ATPase and chaperone activities of Hsp90, caused by geraniin binding, induce cytotoxic effects in the tumor cell lines tested, with a large percentage of cells containing hypodiploid DNA. These results are in agreement with those reported in human melanoma cells, the activation of caspase-8, the cleavage of Bid, and the induction of cytochrome.
analysis, the tannin geraniin was identified as an efficient ligand of Hsp90a, showing a high affinity for this chaperone, similar to that found for 17-AAG and derivates. The comparison of the HSP90a proteolytic patterns in the presence or absence of geraniin indicated that this compound binds at the Nterminus of the chaperone, as reported for several Hsp90 inhibitors. Through targeting the ATP-binding site of the N-terminal domain, the inhibitors probably prevent Hsp90 from forming a closed N-terminal dimeric state and, consequently, alter the chaperone activity of the molecule. This mechanism was proved for geraniin, which was able to reduce Hsp90 chaperone activity more efficiently than 17-AAG. The impaired ATPase and chaperone activities of Hsp90, caused by geraniin binding, induce cytotoxic effects in the tumor cell lines tested, with a large percentage of cells containing hypodiploid DNA. These results are in agreement with those reported in human melanoma cells, the activation of caspase-8, the cleavage of Bid, and the induction of cytochrome.
These naturally-occurring intracellular peptides modulate cellular activities based on the finding that synthetic peptides
Amino acids can mimic or block protein functions and produce physiological changes in cellular function. In some cases, the VE-822 synthetic peptides used to produce cellular changes corresponded to peptides found in peptidomics analyses of the tissue. For example, the addition of specific peptides was shown to modulate the signal transduction elicited by agonists of G-protein coupled receptors in HEK293 and CHO cells. Intracellular peptides derived from rat adipose tissue proteins facilitate insulin-induced glucose uptake in 3T3-L1 adipocytes. In C.elegans, peptides produced from mitochondrial proteins were shown to signal nuclear-encoded mitochondrial chaperone genes and indicate the stress of mitochondrial protein misfolding. In Drosophila, peptides encoded by small open reading frame genes were found to control epidermal differentiation by modifying the activity of transcription factors. Thus, an emerging concept is that peptides produced from cytosolic, mitochondrial, and/or nuclear proteins have functional roles in cellular processes, and are not merely intermediates in the protein degradation pathway. There are four major peptide-generating systems within cells: proteasomes, calpains, caspases, and lysosomes. The LY2157299 TGF-beta inhibitor proteasome complex plays a major role in protein turnover, degrading proteins into peptides of 4�C25 amino acids with an average size around 10 amino acids. Calpains are a family of calcium-regulated proteases that perform limited proteolysis. Caspases are also a family of intracellular proteases, but with a strict substrate specificity for cleavage at sites containing an Asp residue. Lysosomes are organelles that degrade proteins by a series of endo- and exopeptidase activities. In addition to these proteases, a number of cytosolic oligopeptidases exist, including thimet oligopeptidase, neurolysin, post-prolyl oligopeptidase, nardilysin, and insulin degrading enzyme. These oligopeptidases are not capable of cleaving proteins; they selectively cleave a subset of cellular peptides into smaller fragments. Degradation of intracellular peptides into amino acids occurs through the action of aminopeptidases and other enzymes. Previous studies aimed at determining the proteolytic system involved in producing the intracellular peptides of human embryonic kidney 293Tcells implicated the proteasome complex and not calpains based on the observation that epoxomicinbut not A23187affected intracellular peptide levels. Epoxomicin is an irreversible inhibitor of the proteasome, potently inhibiting the beta-5 subunitand less potently inhibiting the beta-2 subunit. Consistent with this activity of epoxomicin, most of the intracellular peptides that resulted from protein cleavage at hydrophobic sites were greatly reduced by 0.2 mM epoxomicin while those peptides that resulted from protein cleavage at basic amino acids were reduced by 2 mM epoxomicin but not by 0.2 mM epoxomicin. Furthermore, many of the intracellular peptides that resulted from cleavage at beta-1 siteswere elevated by epoxomicin treatment; this is consistent with the idea that proteins transported into the epoxomicin-inhibited proteasome cannot be cleaved at their normal sitesand as a result there is increased activity at alternate sites. Bortezomib has 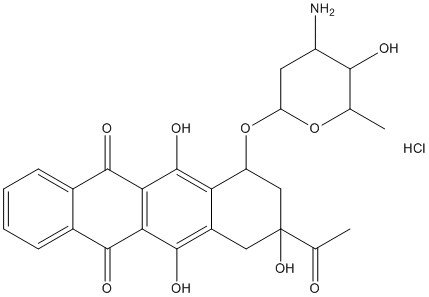 been reported to be a highly selective proteasome inhibitor with greatest potency for the beta-5 subunit and lower potency for the beta-1 subunit. Bortezomib has been successful for the treatment of several types of cancer, including multiple myeloma. A major side effect of bortezomib is neuropathy, presumably due to the action of the drug on nerve cells. In the present study, we tested the effect of bortezomib on levels of peptides in two different.
been reported to be a highly selective proteasome inhibitor with greatest potency for the beta-5 subunit and lower potency for the beta-1 subunit. Bortezomib has been successful for the treatment of several types of cancer, including multiple myeloma. A major side effect of bortezomib is neuropathy, presumably due to the action of the drug on nerve cells. In the present study, we tested the effect of bortezomib on levels of peptides in two different.
Stabilization of the tetrahedral intermediate observed peptides do not match the caspase consensus site
It is also possible that calpain-mediated cleavage of proteins is elevated by bortezomib; this has been proposed to explain the increased degradation of IkBa caused by bortezomib high content screening treatment of various human cell lines. However, a previous peptidomics study did not detect major changes in levels of intracellular peptides when SH-SY5Y cells were treated with a calcium ionophore known to activate calpains. Another possibility is activation of autophagy by bortezomib, which is known to induce autophagy in several systems. This idea is attractive because of the large number of mitochondrial protein fragments found to be elevated by bortezomib. However, the standard marker for autophagy, LC3, showed no evidence of autophagy upon treatment of HEK293T or SH-SY5Y cells with high concentrations of bortezomib for 1 hour. Furthermore, treatment of cells with rapamycin for 1 hour to produce autophagy had little effect on the cellular peptides. Taken together, these results suggest that autophagy does not contribute to the altered peptidome observed upon treatment of cells with bortezomib for short time periods. A third possibility to explain  the increase in many peptides is that protein levels are induced by bortezomib. A previous RNA microarray analysis found that thousands of mRNAs were either up- or down-regulated by treatment with bortezomib for 14, 24, or 48 hours. A cross-comparison of the RNA microarray study and our results found no correlation between those proteins corresponding to up-regulated peptides and mRNA changes at 14 and 24 hour time points. Furthermore, our finding that most of the peptides were up-regulated as early as 30 minutes after the start of the exposure to bortezomib argues against a general effect on protein synthesis; while the synthesis of some proteins may be stimulated within 30 minutes of the start of bortezomib treatment, it is unlikely that all of the affected proteins will show such a rapid increase. Furthermore, a change in protein levels would not explain why all peptides derived from a particular protein are not similarly affected. For example, although many fragments of heat shock 10 kDa protein 1are elevated, one fragment is significantly decreased by 50 and 500 nM bortezomib. Similar variability in the changes of peptides derived from other proteins is observed. Therefore, an increase in the synthesis of these proteins would not explain why some of the peptides derived from these proteins were decreased by the bortezomib treatment. A fourth possibility is that bortezomib BIBW2992 interferes with the further degradation of the peptides produced by the proteasome. Although bortezomib is usually described in the literature as being highly specific for the proteasome with no off-target effects, a recent study has shown that bortezomib inhibits serine proteases such as cathepsins A and G, chymase, dipeptidyl peptidase II, and HtrA2/Omi. Although none of these enzymes are thought to function in the degradation of peptides produced by the proteasome, it is possible that bortezomib has additional off target effects and inhibits a cytosolic peptidase. This possibility would be consistent with the increase in peptides derived from cytosolic proteins as well as mitochondrial proteins; this organelle contains transporters that export peptides generated by proteases within the mitochondria, and these peptides are subsequently degraded by cytosolic peptidases. The mechanism of action of bortezomib as an antitumor agent is thought to involve a reduction in protein turnover and/or protein activation, especially for proteins such as NFkB and cyclins. To generate the active NFkB transcriptional dimeric complexes, two precursors, NFkB1and NFkB2, have to undergo limited proteolytic processing by the proteasome to yield.
the increase in many peptides is that protein levels are induced by bortezomib. A previous RNA microarray analysis found that thousands of mRNAs were either up- or down-regulated by treatment with bortezomib for 14, 24, or 48 hours. A cross-comparison of the RNA microarray study and our results found no correlation between those proteins corresponding to up-regulated peptides and mRNA changes at 14 and 24 hour time points. Furthermore, our finding that most of the peptides were up-regulated as early as 30 minutes after the start of the exposure to bortezomib argues against a general effect on protein synthesis; while the synthesis of some proteins may be stimulated within 30 minutes of the start of bortezomib treatment, it is unlikely that all of the affected proteins will show such a rapid increase. Furthermore, a change in protein levels would not explain why all peptides derived from a particular protein are not similarly affected. For example, although many fragments of heat shock 10 kDa protein 1are elevated, one fragment is significantly decreased by 50 and 500 nM bortezomib. Similar variability in the changes of peptides derived from other proteins is observed. Therefore, an increase in the synthesis of these proteins would not explain why some of the peptides derived from these proteins were decreased by the bortezomib treatment. A fourth possibility is that bortezomib BIBW2992 interferes with the further degradation of the peptides produced by the proteasome. Although bortezomib is usually described in the literature as being highly specific for the proteasome with no off-target effects, a recent study has shown that bortezomib inhibits serine proteases such as cathepsins A and G, chymase, dipeptidyl peptidase II, and HtrA2/Omi. Although none of these enzymes are thought to function in the degradation of peptides produced by the proteasome, it is possible that bortezomib has additional off target effects and inhibits a cytosolic peptidase. This possibility would be consistent with the increase in peptides derived from cytosolic proteins as well as mitochondrial proteins; this organelle contains transporters that export peptides generated by proteases within the mitochondria, and these peptides are subsequently degraded by cytosolic peptidases. The mechanism of action of bortezomib as an antitumor agent is thought to involve a reduction in protein turnover and/or protein activation, especially for proteins such as NFkB and cyclins. To generate the active NFkB transcriptional dimeric complexes, two precursors, NFkB1and NFkB2, have to undergo limited proteolytic processing by the proteasome to yield.