For this reason, the protective effect of CQ in the murine EBOV challenge model is encouraging. None of the reported studies address the pharmacodynamics of the antiviral activity by demonstrating that the compound accumulates in the relevant tissue or compartments where the virus is replicating in vivo. Chloroquine has a large volume of distribution, which Temozolomide suggests that its rapid dissemination into extravascular tissues may impact its inhibitory activity. Clearly, the spectrum of viruses for which this class of compounds would be Reversine useful in vivo will be strongly determined by this factor, as well as by the potency of the compound itself in inhibiting specific steps in viral replication. Improvements in formulation, such as encapsulation within liposomes may also be of utility in modifying the pharmacokinetics of 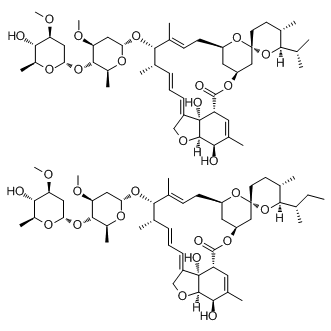 CQ in vivo. The antiviral activity of CQ may serve as an initial starting point for antiviral development through optimization of the 4AQ scaffold and by exploiting the decades of experience in toxicological investigation for this class of compounds. Significant effort has been expended in optimizing derivatives of CQ for malaria strains that have acquired resistance. By optimizing the antiviral activity of these compounds for short- or intermediate-term therapeutic dosing, it should be possible to develop analogs with entirely different properties than those required for antimalarial activity, including lower toxicity. We have successfully identified many clinically useful drugs that are potential inhibitors of bacteria and virus infection. The efficacy of lomefloxacin against BA and CQ against EBOV in vivo has not been previously reported. The ability of erythromycin to inhibit filoviruses as well as bacteria is intriguing and suggests that this drug can act not only by impacting bacterial growth but also on the cell itself, possibly by altering uptake of the pathogen. Many other pathogen-specific drugs were identified that will require evaluation in animal models. The identification of these compounds lends credence to the repurposing approach for novel drug discovery against high containment and/or biodefenserelated pathogens. The potential to reduce the time from bench to clinic is great, and accelerating this process would save lives in the event of an outbreak of any pathogen. In the past decade, Phosphatase of Regenerating Liver family members have been touted as molecular markers that significantly correlate to the ability of cancers to metastasize,,. In addition, laboratory studies indicate that PRLs are promising therapeutic targets; interfering with PRL function using antibodies and RNA interference has shown dramatic reduction in tumor formation in mice,. PRL-1 was first isolated as a novel tyrosine phosphatase that is immediately transcribed following a partial hepatectomy, continually expressed in a number of tumor cell lines and able to transform non-tumorigenic cells,. Later, PRL-2 and PRL-3 were identified by sequence analysis. Studies in cell culture indicate that exogenous expression of PRLs can induce cell proliferation,,,, migration,,, and invasiveness,,. Most significantly, constitutive expression of PRL1 and -3 enable cultured cells to form tumors when injected into mice,,. The potential of increased levels of PRLs to actively contribute to oncogenesis complements dozens of studies correlating PRL expression to tumor aggressiveness.
CQ in vivo. The antiviral activity of CQ may serve as an initial starting point for antiviral development through optimization of the 4AQ scaffold and by exploiting the decades of experience in toxicological investigation for this class of compounds. Significant effort has been expended in optimizing derivatives of CQ for malaria strains that have acquired resistance. By optimizing the antiviral activity of these compounds for short- or intermediate-term therapeutic dosing, it should be possible to develop analogs with entirely different properties than those required for antimalarial activity, including lower toxicity. We have successfully identified many clinically useful drugs that are potential inhibitors of bacteria and virus infection. The efficacy of lomefloxacin against BA and CQ against EBOV in vivo has not been previously reported. The ability of erythromycin to inhibit filoviruses as well as bacteria is intriguing and suggests that this drug can act not only by impacting bacterial growth but also on the cell itself, possibly by altering uptake of the pathogen. Many other pathogen-specific drugs were identified that will require evaluation in animal models. The identification of these compounds lends credence to the repurposing approach for novel drug discovery against high containment and/or biodefenserelated pathogens. The potential to reduce the time from bench to clinic is great, and accelerating this process would save lives in the event of an outbreak of any pathogen. In the past decade, Phosphatase of Regenerating Liver family members have been touted as molecular markers that significantly correlate to the ability of cancers to metastasize,,. In addition, laboratory studies indicate that PRLs are promising therapeutic targets; interfering with PRL function using antibodies and RNA interference has shown dramatic reduction in tumor formation in mice,. PRL-1 was first isolated as a novel tyrosine phosphatase that is immediately transcribed following a partial hepatectomy, continually expressed in a number of tumor cell lines and able to transform non-tumorigenic cells,. Later, PRL-2 and PRL-3 were identified by sequence analysis. Studies in cell culture indicate that exogenous expression of PRLs can induce cell proliferation,,,, migration,,, and invasiveness,,. Most significantly, constitutive expression of PRL1 and -3 enable cultured cells to form tumors when injected into mice,,. The potential of increased levels of PRLs to actively contribute to oncogenesis complements dozens of studies correlating PRL expression to tumor aggressiveness.
Category: GPCR Compound Library
The more straightforward model of dPRL-1 simply countering activation of Src was also not supported by our studies
This results in activation of Akt, which is well established as protecting cells against apoptosis and also promoting cell migration,. Interestingly, inhibition of Akt has also been shown to be a key player for PRL-3 to arrest cells. Experimenting with levels of PRL-3 overexpression appears to reconcile the opposing effects of PRL-3 on Akt; Basak et al., could detect activation of Akt in response to PRL-3, but only transiently, until level of PRL-3 became highly elevated. Although there is a rapidly growing amount of literature on the mammalian family of PRL phosphatases, several studies have conflicting results. These studies each examine PRL in a different genetic environment, which may mean modulators and effectors of PRL localization or function are missing or mutated. Our study using Drosophila is the first to examine overexpressed PRL in genetically controlled animal model. This system confirms that PRL can function as a growth inhibitor under normal and oncogenic conditions that can be dependent on subGDC-0449 Hedgehog inhibitor membrane distribution. SB203580 dPRL-1 is a ubiquitously expressed protein found in both proliferating and differentiated tissues of Drosophila that can function as a growth inhibitor at elevated levels. Our 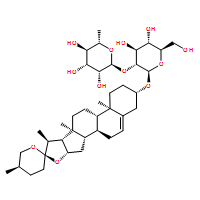 work supports the model that other cellular alterations are required for elevated levels of PRL to promote cancer. For example, because the CAAX motif is required for dPRL-1 to suppress growth, cellular modifications that interfere with the motif could be one means towards enabling PRLs to act as oncogenes instead. Indeed, our analysis of endogenous dPRL-1 expression during embryogenesis demonstrated that dPRL-1 levels can be high in the cytoplasm in spite of an intact CAAX motif, suggesting other proteins can override CAAXdriven membrane localization. While others�� work has highlighted the need of the CAAX motif for PRLs function, we are the first to see that at least one member of the PRL family can still associate with the plasma membrane without CAAX. This association may occur through the polybasic region adjacent to CAAX, which has been shown to be required for membrane association in addition to CAAX. Our work is also the first to report the accumulation of a PRL family member to apico-lateral locations in epithelial cells; suggesting that dPRL-1 is forming stable interactions with other membrane-bound proteins. Intriguingly, we found that elevated levels of dPRL-1 can have opposing outcomes in genetic backgrounds expressing known oncogenes; resulting in synergistic lethality with Ras but rescuing Src-induced lethality. Src overexpression likely results in lethality because the massively overgrown wing disc becomes developmentally disorganized. While dPRL-1 effectively inhibits Src-induced overgrowth, another mechanism to counter Src function must exist because dPRL-1NC, which does not inhibit growth under normal levels of Src, retains the ability to counter Src-induced lethality. One possibility was that dPRL-1/dPRL-1NC could increase apoptosis, thus eliminating excess tissue. Furthermore, this phenotype could be accomplished by dPRL-1 leading to an increase in Src activity as has been seen in mammalian studies,,. Previous studies in Drosophila have shown a dose response with lower levels of Src leading to proliferation but higher levels resulting in apoptosis. However, we did not detect elevated levels of apoptosis in animals overexpressing both dPRL-1 and Src.
work supports the model that other cellular alterations are required for elevated levels of PRL to promote cancer. For example, because the CAAX motif is required for dPRL-1 to suppress growth, cellular modifications that interfere with the motif could be one means towards enabling PRLs to act as oncogenes instead. Indeed, our analysis of endogenous dPRL-1 expression during embryogenesis demonstrated that dPRL-1 levels can be high in the cytoplasm in spite of an intact CAAX motif, suggesting other proteins can override CAAXdriven membrane localization. While others�� work has highlighted the need of the CAAX motif for PRLs function, we are the first to see that at least one member of the PRL family can still associate with the plasma membrane without CAAX. This association may occur through the polybasic region adjacent to CAAX, which has been shown to be required for membrane association in addition to CAAX. Our work is also the first to report the accumulation of a PRL family member to apico-lateral locations in epithelial cells; suggesting that dPRL-1 is forming stable interactions with other membrane-bound proteins. Intriguingly, we found that elevated levels of dPRL-1 can have opposing outcomes in genetic backgrounds expressing known oncogenes; resulting in synergistic lethality with Ras but rescuing Src-induced lethality. Src overexpression likely results in lethality because the massively overgrown wing disc becomes developmentally disorganized. While dPRL-1 effectively inhibits Src-induced overgrowth, another mechanism to counter Src function must exist because dPRL-1NC, which does not inhibit growth under normal levels of Src, retains the ability to counter Src-induced lethality. One possibility was that dPRL-1/dPRL-1NC could increase apoptosis, thus eliminating excess tissue. Furthermore, this phenotype could be accomplished by dPRL-1 leading to an increase in Src activity as has been seen in mammalian studies,,. Previous studies in Drosophila have shown a dose response with lower levels of Src leading to proliferation but higher levels resulting in apoptosis. However, we did not detect elevated levels of apoptosis in animals overexpressing both dPRL-1 and Src.
These excluded volumes attempt to penalize molecules occupying steric regions that are not occupied by active molecules
Second, the pharmacophore models are ranked based on a measure of sensitivity and specificity and the top models are returned. The pharmacophore models are enumerated and then the selectivity is estimated based on a Genetic Function Approximation GFA) model. The GFA model for the selectivity of a pharmacophore is built from a training set of 3285 pharmacophore models. This set is used for searching the CapDiverse database in DS. The logarithmic values of the number of database search hits are  used as the targets. The number of total features in pharmacophore models and the feature-feature distance bin values are used as the descriptors for training the GFA model. Molecular docking studies were carried out using GOLD 5.1 program from Cambridge Crystallographic Data Center, UK. GOLD uses a genetic algorithm for docking ligands into protein binding sites to explore the full range of ligand conformational flexibility with partial flexibility of protein. Molecular docking was performed to generate the bioactive binding poses of inhibitors in the active site of enzyme. Protein coordinates from the Niltubacin crystal structure of chymase co-crystallized with N7O, determined at a resolution of 1.8A ? were used to define the active site. All the water molecules present in the protein structure were removed and hydrogen atoms were added. The active site was defined with a 10 A ? radius around the ligand present in the crystal structure. Ten docking runs were performed per structure unless three of the 10 poses were within 1.5 A ? RMSD of each other. All the hit compounds as well as training set compounds were docked into chymase binding site. The GOLD fitness score is calculated from the contributions of hydrogen bond and van der Waals interactions between the protein and ligand, intramolecular hydrogen bonds and strains of the ligand. The interacting ability of a compound depends on the fitness score, greater the GOLD fitness score better the binding affinity. The protein – ligand interactions were examined by DS. Hit molecules which showed the expected interactions with the critical amino acids present in the active site of the protein, and comparable high binding scores than the bound ligand, were selected as potent inhibitors of chymase. The experimentally known and highly active chymase inhibitors with substantial structural diversity which were used for the common feature pharmacophore generation were selected for DFT calculations. Moreover, four final hits KM09155, HTS00581, HTS0589, and Compound1192 retrieved from databases by the selected pharmacophore models, which showed important results with respect to all Z-VAD-FMK Caspase inhibitor properties like key molecular interactions with the active site components, calculated drug-like properties, and high GOLD fitness score, were also designated for DFT study. Various quantum-chemical descriptors such as LUMO, HOMO, and locations of molecular electrostatic potentials were computed. In this study, four different 3D structures of chymase bound with its inhibitors such as 3N7O, 1T31, 3SON, and 2HVX were selected as input for structure-based pharmacophore generation. The generated four pharmacophore models along with their excluded volume spheres and geometrical constrain are illustrated in Figure 4. The excluded volume spheres presented in our models provide an insight regarding the disallowed regions in the binding site.
used as the targets. The number of total features in pharmacophore models and the feature-feature distance bin values are used as the descriptors for training the GFA model. Molecular docking studies were carried out using GOLD 5.1 program from Cambridge Crystallographic Data Center, UK. GOLD uses a genetic algorithm for docking ligands into protein binding sites to explore the full range of ligand conformational flexibility with partial flexibility of protein. Molecular docking was performed to generate the bioactive binding poses of inhibitors in the active site of enzyme. Protein coordinates from the Niltubacin crystal structure of chymase co-crystallized with N7O, determined at a resolution of 1.8A ? were used to define the active site. All the water molecules present in the protein structure were removed and hydrogen atoms were added. The active site was defined with a 10 A ? radius around the ligand present in the crystal structure. Ten docking runs were performed per structure unless three of the 10 poses were within 1.5 A ? RMSD of each other. All the hit compounds as well as training set compounds were docked into chymase binding site. The GOLD fitness score is calculated from the contributions of hydrogen bond and van der Waals interactions between the protein and ligand, intramolecular hydrogen bonds and strains of the ligand. The interacting ability of a compound depends on the fitness score, greater the GOLD fitness score better the binding affinity. The protein – ligand interactions were examined by DS. Hit molecules which showed the expected interactions with the critical amino acids present in the active site of the protein, and comparable high binding scores than the bound ligand, were selected as potent inhibitors of chymase. The experimentally known and highly active chymase inhibitors with substantial structural diversity which were used for the common feature pharmacophore generation were selected for DFT calculations. Moreover, four final hits KM09155, HTS00581, HTS0589, and Compound1192 retrieved from databases by the selected pharmacophore models, which showed important results with respect to all Z-VAD-FMK Caspase inhibitor properties like key molecular interactions with the active site components, calculated drug-like properties, and high GOLD fitness score, were also designated for DFT study. Various quantum-chemical descriptors such as LUMO, HOMO, and locations of molecular electrostatic potentials were computed. In this study, four different 3D structures of chymase bound with its inhibitors such as 3N7O, 1T31, 3SON, and 2HVX were selected as input for structure-based pharmacophore generation. The generated four pharmacophore models along with their excluded volume spheres and geometrical constrain are illustrated in Figure 4. The excluded volume spheres presented in our models provide an insight regarding the disallowed regions in the binding site.
There remains an unmet need for effective vaccines against the diseases transmitted by Iscapularis ticks
Tick-based vaccine molecules that can block the transmission of multiple pathogens are desired, and would have an advantage over pathogen-based vaccines that target individual pathogens. Since tick feeding is intimately intertwined with pathogen transmission and acquisition, MK-4827 PARP inhibitor research efforts have focused on identifying tick molecules critical for tick feeding. The emphasis has been on tick salivary proteins that suppress and modulate host defense and haemostatic mechanisms, and impair the ability of the host to thwart tick feeding. However, the functional redundancy and structural paralogy inherent in the I. scapularis salivary gland transcriptome, and proteome has confounded the development of viable salivary vaccine targets to effectively block tick feeding. Ixodid ticks feed for 4�C10 days, and blood in the gut is maintained in a fluid state throughout the process of repletion, and up to 24�C48 h beyond repletion. The anticoagulation mechanisms in the gut have not been addressed at the molecular level. Ticks alternately deposit saliva and suck blood at the tick bite site. It is therefore presumed that tick salivary anticoagulants deposited into the tick bite site are taken up along with the blood, and function both at the vector-host interface and in the tick gut to keep the blood fluid. We now present data to show that the tick gut is not a passive bystander, and that it plays an active role in thwarting host coagulation. We show that the tick gut expresses a thrombin inhibitor, Ixophilin, during tick feeding. These findings open up a new avenue of research, hitherto ignored, that can increase our understanding of tick feeding strategies, and  provide novel targets for interrupting tick feeding and pathogen transmission. Tick anticoagulation strategies are central to successful feeding and it is now well documented that the tick utilizes a multipronged strategy to thwart host hemostasis. However, the functional redundancy of the salivary “anticoagulome” poses a major bottleneck in efforts to develop vaccines targeting specific salivary anticoagulants. We now shift the focus from the tick saliva to the tick gut and draw attention to the role of the tick gut in anticoagulation and reveal a new critical aspect in tick feeding. Ixodid ticks feed for 4�C10 days and imbibe as much as 100 times their body weight of blood meal during engorgement. The tick gut serves as a storage organ for fluid blood conducive for both receptor-mediated and fluid-phase LEE011 endocytosis of the blood meal by the gut digestive cells. The blood in the gut is maintained in a fluid state throughout the process of repletion and up to 24�C48 hours beyond repletion. Ticks alternately deposit saliva into and suck blood from the tick bite site, and it was presumed that salivary immune modulators and anticoagulants deposited into the bite site, and absorbed into the gut along with the bloodmeal might provide essential functions both at the bite site and in the gut. However, a recent analysis of the gut transcriptome of Dermacentor variabilis by Anderson et al showed that about 6% of the sequenced transcripts encoded secreted proteins with putative antioxidant, anticoagulant and antimicrobial functions distinct from that observed in salivary glands. Consistent with this emerging active role for the tick gut in feeding, several reports demonstrate the expression of gut-specific anticoagulants in several Ixodid ticks.
provide novel targets for interrupting tick feeding and pathogen transmission. Tick anticoagulation strategies are central to successful feeding and it is now well documented that the tick utilizes a multipronged strategy to thwart host hemostasis. However, the functional redundancy of the salivary “anticoagulome” poses a major bottleneck in efforts to develop vaccines targeting specific salivary anticoagulants. We now shift the focus from the tick saliva to the tick gut and draw attention to the role of the tick gut in anticoagulation and reveal a new critical aspect in tick feeding. Ixodid ticks feed for 4�C10 days and imbibe as much as 100 times their body weight of blood meal during engorgement. The tick gut serves as a storage organ for fluid blood conducive for both receptor-mediated and fluid-phase LEE011 endocytosis of the blood meal by the gut digestive cells. The blood in the gut is maintained in a fluid state throughout the process of repletion and up to 24�C48 hours beyond repletion. Ticks alternately deposit saliva into and suck blood from the tick bite site, and it was presumed that salivary immune modulators and anticoagulants deposited into the bite site, and absorbed into the gut along with the bloodmeal might provide essential functions both at the bite site and in the gut. However, a recent analysis of the gut transcriptome of Dermacentor variabilis by Anderson et al showed that about 6% of the sequenced transcripts encoded secreted proteins with putative antioxidant, anticoagulant and antimicrobial functions distinct from that observed in salivary glands. Consistent with this emerging active role for the tick gut in feeding, several reports demonstrate the expression of gut-specific anticoagulants in several Ixodid ticks.
The prevailing paradigm proposes the bone as the as demonstrated here and elsewhere with gene activation
Recently, it has also been shown that only a minority of DAC-mediated demethylated promoters are associated with nucleosome remodelling. Chromatin remodelling is required for gene reactivation after DNA demethylation as induced by DAC treatment and the combination of DNMT and histone deacetylase inhibitors has been shown to induce re-expression of tumour suppressor genes in ovarian and colon cancer cell cultures. A phase I study of DAC in combination with suberoylanilide hydroamic acid in patients with a range of tumour types has been reported. We show here that CGI demethylation is not generally Afatinib sufficient to change gene expression. However, it may change the epigenetic niche providing a permissive environment for histone remodelling. In this study, we have established an in vitro model of the epigenetic modification following prolonged treatment of demethylating agents. Since the effect was maintained after the cessation of treatment, it may provide a useful tool for testing the effects of histone modifying agents in a reduced DNA methylation environment. The data-set provided with this work provides a rich resource for further analysis related to both DNA methylation in general,  the effect of demethylating agents at pharmacological dosages and to the epigenetic changes that underlie myelodysplastic syndrome. We believe that the full value of this can only be realised in combination with clinical data and we present it here as to make it available for further analysis. Fibroblast growth factor 23 is a phosphaturic hormone produced in response to an increase in phosphorus load or high levels of calcitriol or parathyroid hormone. FGF23 acts by inducing renal phosphate excretion by kidney proximal tubular cells through reduction of the expression of type 2a and 2c sodium phosphate co-transporters. FGF23 also suppresses the production of vitamin D��s active form in the kidney by inhibiting the synthetic enzyme 1a-hydroxylase, thereby acting as counter-regulatory hormone for vitamin D. The reduction in circulating 1,25dihydroxyvitamin D levels by FGF23 contributes to cause negative phosphate balance through limiting phosphate absorption from the intestine. FGF23 exerts its intrarenal biological function by binding to cognate FGF receptors requiring the presence of Klotho, a transmembrane protein highly expressed in the kidney, as a co-receptor. The site of synthesis of FGF23 is primarily the bone tissue, more specifically osteocytes and osteoblasts, although FGF23 is also espressed by brain, thymus, liver, spleen and heart. Since the kidney is an important target of FGF23, and the circulating levels of FGF23 have been found to increase in association with disease progression and cardiovascular events in chronic kidney disease and diabetic nephropathy, we wondered whether the kidney could be a source of FGF23 during the development of renal disease. Few data are so far available showing that renal tissue expressed FGF23 at very low level, if any, in normal conditions and in uremic rats. We took advantage of the Zucker diabetic fatty rat model of human type 2 diabetic nephropathy characterized by obesity, hyperlipidemia, insulin resistance, progressive renal injury and cardiac abnormalities and evaluated the expression of FGF23 in the kidney during the course of the disease. We also investigated whether renoprotective effects of ACE inhibitor in this model were associated with modulation of renal FGF23 and Klotho expression. The present study shows that the kidney of ZDF rats, a model resembling human type 2 diabetic nephropathy, expressed FGF23 starting from the age of 4 months when rats have already Nutlin-3 significant levels of proteinuria and signs of renal injury. FGF23 mRNA expression further increased with time as diabetic disease progressed, reaching levels that were 3�C4 times higher than at 4 months. To our knowledge this is the first evidence of FGF23 production by the kidney during renal disease progression. Upregulation of FGF23 mRNA resulted in the expression of the corresponding protein localized at proximal and distal tubules in focal areas of the kidney. A previous study by Mirams et al that analyzed FGF23 mRNA expression in several human tissues showed that FGF23 was detectable in kidney tissue at low levels. Actually, the highest expression was found in bone followed by kidney medulla, liver, thyroid and kidney cortex. FGF23 bone expression was approximately 30 times higher than renal expression in normal conditions, but no data are available that compared tissues from patients with renal disease.
the effect of demethylating agents at pharmacological dosages and to the epigenetic changes that underlie myelodysplastic syndrome. We believe that the full value of this can only be realised in combination with clinical data and we present it here as to make it available for further analysis. Fibroblast growth factor 23 is a phosphaturic hormone produced in response to an increase in phosphorus load or high levels of calcitriol or parathyroid hormone. FGF23 acts by inducing renal phosphate excretion by kidney proximal tubular cells through reduction of the expression of type 2a and 2c sodium phosphate co-transporters. FGF23 also suppresses the production of vitamin D��s active form in the kidney by inhibiting the synthetic enzyme 1a-hydroxylase, thereby acting as counter-regulatory hormone for vitamin D. The reduction in circulating 1,25dihydroxyvitamin D levels by FGF23 contributes to cause negative phosphate balance through limiting phosphate absorption from the intestine. FGF23 exerts its intrarenal biological function by binding to cognate FGF receptors requiring the presence of Klotho, a transmembrane protein highly expressed in the kidney, as a co-receptor. The site of synthesis of FGF23 is primarily the bone tissue, more specifically osteocytes and osteoblasts, although FGF23 is also espressed by brain, thymus, liver, spleen and heart. Since the kidney is an important target of FGF23, and the circulating levels of FGF23 have been found to increase in association with disease progression and cardiovascular events in chronic kidney disease and diabetic nephropathy, we wondered whether the kidney could be a source of FGF23 during the development of renal disease. Few data are so far available showing that renal tissue expressed FGF23 at very low level, if any, in normal conditions and in uremic rats. We took advantage of the Zucker diabetic fatty rat model of human type 2 diabetic nephropathy characterized by obesity, hyperlipidemia, insulin resistance, progressive renal injury and cardiac abnormalities and evaluated the expression of FGF23 in the kidney during the course of the disease. We also investigated whether renoprotective effects of ACE inhibitor in this model were associated with modulation of renal FGF23 and Klotho expression. The present study shows that the kidney of ZDF rats, a model resembling human type 2 diabetic nephropathy, expressed FGF23 starting from the age of 4 months when rats have already Nutlin-3 significant levels of proteinuria and signs of renal injury. FGF23 mRNA expression further increased with time as diabetic disease progressed, reaching levels that were 3�C4 times higher than at 4 months. To our knowledge this is the first evidence of FGF23 production by the kidney during renal disease progression. Upregulation of FGF23 mRNA resulted in the expression of the corresponding protein localized at proximal and distal tubules in focal areas of the kidney. A previous study by Mirams et al that analyzed FGF23 mRNA expression in several human tissues showed that FGF23 was detectable in kidney tissue at low levels. Actually, the highest expression was found in bone followed by kidney medulla, liver, thyroid and kidney cortex. FGF23 bone expression was approximately 30 times higher than renal expression in normal conditions, but no data are available that compared tissues from patients with renal disease.