Here, using complementary molecular techniques, we demonstrated the conservation of the coding region of BDNF and Ntrk2 across several mammalian species, the mRNA expression of both genes within the uterus, and the uterine localization of both proteins in two species that menstruate, and four that do not. Additionally, we have shown that several protein isoforms of each gene were present in the human uterus, and that the antibodies employed in this study were specific to BDNF and Ntrk2 respectively. BDNF and Ntrk2 are part of the complex messenger system that is the neurotrophins, which regulate several physiological pathways, and thus we suggest are potentially important to uterine function. Our results show that both BDNF and Ntrk2 are highly conserved across the mammalian species studied, with protein sequences having greater homology than mRNA sequences. This was not entirely unexpected, as in some cases multiple codons exist for a single amino acid, and thus a base-pair substitution in the mRNA sequence might not alter the protein. Over time, as each of the species studied evolved, silent mutations in the genes likely arose. During evolution, Gotz et al., suggest that BDNF was more highly conserved than NGF across vertebrates. In our study the PCR primer pairs designed to isolate BDNF and Ntrk2 were capable of doing so in the uterus of all animals, and both antibodies employed in this study demonstrated specific uterine immunoreactivity for BDNF and Ntrk2 in each of the six mammals examined, supporting high sequence homology amongst orthologs over evolution. Doxorubicin Antibody specificity in the current study was ascertained in two ways. Firstly, by ensuring bands of the appropriate size were seen when Western blot was performed with human recombinant BDNF and Ntrk2. Secondly, mouse brain sections were stained for BDNF and Ntrk2 with primary antibodies which had been pre-absorbed with BDNF and Ntrk2, respectively. In sections incubated with pre-absorbed BDNF primary antibodies the staining was less intense than the positive control, which had been stained with anti-BDNF, but more intense than the negative control. Ideally pre-absorption obliterates all staining as the antibody should be completely bound by the excess protein. In the case of pre-absorbed BDNF, some of the BDNF bound to the antiBDNF antibody may have bound to endogenous Ntrk2 receptors, and thus given a faint signal when the secondary antibody was applied. Ntrk2 staining in the mouse brain was obliterated by preabsorption. The results of the antibody specificity tests indicated that the antibodies used for immunohistochemistry and Western blot were specific and capable of detecting BDNF and Ntrk2 within the mammalian uterus. While there are a few studies demonstrating the independent expression of BDNF and Ntrk2 in the uterus, the results of the present  study are the first to show that both ligand and receptor are co-expressed, and co-localized in the uterus of several mammalian species. Our results show BDNF and Ntrk2 expression in the glandular epithelium, luminal epithelium, vascular smooth muscle, and myometrium of the human, mouse, rat, pig, and bat uterus. A GW786034 similar pattern of expression was also observed in the uterus of the pregnant mare. This is the first comprehensive and cross-species comparison of BDNF and Ntrk2 mRNA, and protein in the mammalian uterus.
study are the first to show that both ligand and receptor are co-expressed, and co-localized in the uterus of several mammalian species. Our results show BDNF and Ntrk2 expression in the glandular epithelium, luminal epithelium, vascular smooth muscle, and myometrium of the human, mouse, rat, pig, and bat uterus. A GW786034 similar pattern of expression was also observed in the uterus of the pregnant mare. This is the first comprehensive and cross-species comparison of BDNF and Ntrk2 mRNA, and protein in the mammalian uterus.
Category: GPCR Compound Library
Moreover the uterine expression of BDNF and Ntrk2 has not been examined in species
Furthermore, examination of changes in the phosphoproteome of rape seeds during the filling stage identified 70 phosphoproteins and 16 non-redundant phosphoproteins, which were verified by mapping the phosphorylation sites. Analysis of vacuolar and cell membranes of rice bud and root revealed 230 membrane and membrane-associated proteins, 20% of which are phosphorylated. In addition, soybean root hairs contain 1625 unique phosphopeptides, including 1659 nonredundant phosphorylation sites, which originate from 1126 phosphoproteins. A recent study identified multiple components of ABA-responsive protein phosphorylation network by integrating genetics with phosphoproteomics. Furthermore, Wang et al. have shown the role of the SnRK2 protein kinases in the ABA signaling pathway by using quantitative phosphopro teomics. To date, only a few plant species have been annotated with protein phosphorylation data and these do not include the economically important plant, cotton. We previously examined the proteome of cotton leaves in response to NO treatment. To follow-up this research, we performed quantitative time-course measurements of the phosphoproteome of cotton leaves treated with sodium nitroprusside, a NO donor. This is the first study to show that NO exposure in leaf tissue alters the phosphorylation states of multiple proteins found in cotton. This information should accelerate research on NO metabolic regulation and will lay a novel, theoretical foundation for further related studies in cotton. The neurotrophins are classically known for their participation in the Staurosporine development, growth, function, and survival of neurons in both the central and peripheral GSI-IX nervous system. Although abundant in the nervous system, BDNF and Ntrk2 are expressed in other cell types and tissues, and BDNF mRNA is found in the majority of the human body organs. In humans, mature BDNF is sequestered in platelets and released upon their degranulation. As such, BDNF has access to all tissues and organs. Motile cells including activated T cells, B cells, and monocytes have been shown to express BDNF in vitro, as have eosinophils, dendritic cells, and endothelial cells. In mice, the visceral epithelium, and airway epithelium are significant sources of BDNF. As for Ntrk2, a comprehensive analysis of Ntrk2 immunoreactivity was assessed and it was found to be expressed mainly in glandular cells of the salivary gland, small intestine, colon, endocrine pancreas, bone marrow hematopoietic cells, monocytes/macrophages of the lymph nodes and spleen, and in the epidermis. Previous studies have shown 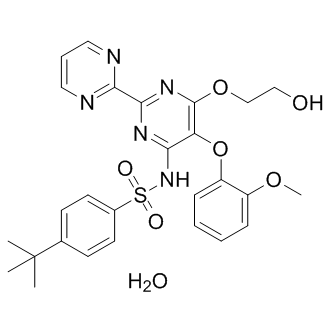 that neurotrophins in the brain are regulated by neuronal activity, and steroid hormones, and that tissue-specific expression is driven by multiple promoters. While BDNF and Ntrk2 expression has been demonstrated in some reproductive tissues including the ovary, and placenta, their uterine expression under physiological conditions has been questionable. BDNF expression was demonstrated by immunohistochemistry in the mouse, and human uterus and by in situ hybridization in the mouse, and rat uterus. While Ntrk2 could not be detected in the mouse and human uterus, others have been successful. To date only one study has looked for the presence of both ligand and receptor simultaneously, in the murine uterus.
that neurotrophins in the brain are regulated by neuronal activity, and steroid hormones, and that tissue-specific expression is driven by multiple promoters. While BDNF and Ntrk2 expression has been demonstrated in some reproductive tissues including the ovary, and placenta, their uterine expression under physiological conditions has been questionable. BDNF expression was demonstrated by immunohistochemistry in the mouse, and human uterus and by in situ hybridization in the mouse, and rat uterus. While Ntrk2 could not be detected in the mouse and human uterus, others have been successful. To date only one study has looked for the presence of both ligand and receptor simultaneously, in the murine uterus.
In computational modeling of cellulose protofibril formation simulated the interactions
For further understanding of FF-TEM views of SP600125 cellular membranes and standard FF-TEM nomenclature, see. Clarifying some aspects of FF-TEM imaging will aid the understanding of our results. When shadowing metal is applied from a fixed angle in FF-TEM, the replica appears three dimensional due to the differential interaction of electron dense metal with the changing topography of the sample surface. The shadowing metal accumulates on a raised intramembrane protein or cellular surface on the side proximal to the shadowing source. Relatively tall objects block the deposition of metal on the side opposite to the shadowing source resulting in electrontranslucent, white, ‘shadows’. If an object is nearly flat relative to the overall sample surface it may be almost uniformly highlighted by a thin metal coating that does not obscure it, as seen below for rosettes CSCs on the ES of the Reversine customer reviews plasma membrane. In differentiating TEs, the patterned secondary wall thickenings push down into the cytoplasm and cause undulations in 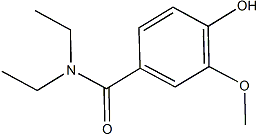 the topography of the plasma membrane. In the lower area of the TE, ‘sw’ marks a plasma membrane trough where the secondary cell wall was completely removed by the fracture process to reveal the plasma membrane ES. In the middle region of the TE, ‘sw’ marks a secondary wall thickening where cell wall material still fills the trough on the left side. A few fibrils, putatively cellulose, remain on the ES of the plasma membrane, and their average diameter was 4.15 nm after subtraction of two times the approximate thickness of the shadowing metal. Sometimes the fibrils appeared irregular on the edges as would be consistent with recent synthesis and nascent crystallization near the extrusion point. Some of the fibrils have hemispherical ends. Other hemispheres are in the vicinity of fibrils, but are not clearly contiguous with one fibril. The average diameter of the hemispheres was 23.9 nm, which is much larger than the typical IMPs scattered throughout the membrane. The hemispheres and the fibrils are shadowed objects that lie on top of the plasma membrane bilayer, but in the same plane. Consistently, all of the images show that the rosette CSCs are nearly flat in the membrane because the six individual globules did not cast shadows. In contrast, some other unidentified IMPs projected higher above the membrane surface and cast white shadows. These observations were consistent with images from other TEs. Often the plasma membrane bilayer splits during fracture and rosette CSCs partition with the protoplasmic fracture face of the plasma membrane as shown in numerous past studies and our work as well. Correspondingly, we sometimes saw holes in the extracellular leaflet of the plasma membrane where the TMHs of CESA had pulled out as the plasma membrane split during fracture. Figure 8B shows another view of a rosette CSC in a small depression of the plasma membrane ES. Figure 8C shows a rosette CSC in the plasma membrane ES in close proximity to a curving fibril about 4 nm wide. Numerous longer fibrils along with a rosette CSC in the plasma membrane ES are shown in Figure 8D. Two fibrils with globular structures at opposite ends are shown in Figure 8E, an observation that is consistent with the bidirectional travel of CESAs that was demonstrated by live-cell imaging during synthesis of epidermal cell walls in hypocotyls.
the topography of the plasma membrane. In the lower area of the TE, ‘sw’ marks a plasma membrane trough where the secondary cell wall was completely removed by the fracture process to reveal the plasma membrane ES. In the middle region of the TE, ‘sw’ marks a secondary wall thickening where cell wall material still fills the trough on the left side. A few fibrils, putatively cellulose, remain on the ES of the plasma membrane, and their average diameter was 4.15 nm after subtraction of two times the approximate thickness of the shadowing metal. Sometimes the fibrils appeared irregular on the edges as would be consistent with recent synthesis and nascent crystallization near the extrusion point. Some of the fibrils have hemispherical ends. Other hemispheres are in the vicinity of fibrils, but are not clearly contiguous with one fibril. The average diameter of the hemispheres was 23.9 nm, which is much larger than the typical IMPs scattered throughout the membrane. The hemispheres and the fibrils are shadowed objects that lie on top of the plasma membrane bilayer, but in the same plane. Consistently, all of the images show that the rosette CSCs are nearly flat in the membrane because the six individual globules did not cast shadows. In contrast, some other unidentified IMPs projected higher above the membrane surface and cast white shadows. These observations were consistent with images from other TEs. Often the plasma membrane bilayer splits during fracture and rosette CSCs partition with the protoplasmic fracture face of the plasma membrane as shown in numerous past studies and our work as well. Correspondingly, we sometimes saw holes in the extracellular leaflet of the plasma membrane where the TMHs of CESA had pulled out as the plasma membrane split during fracture. Figure 8B shows another view of a rosette CSC in a small depression of the plasma membrane ES. Figure 8C shows a rosette CSC in the plasma membrane ES in close proximity to a curving fibril about 4 nm wide. Numerous longer fibrils along with a rosette CSC in the plasma membrane ES are shown in Figure 8D. Two fibrils with globular structures at opposite ends are shown in Figure 8E, an observation that is consistent with the bidirectional travel of CESAs that was demonstrated by live-cell imaging during synthesis of epidermal cell walls in hypocotyls.
Efflux a variety of structurally and functionally diverse chemotherapeutic drugs out of cancer cells
Thereby reducing the intracellular drug accumulation, increasing the likelihood of decreased cytotoxic and thus unsuccessful treatment. Currently, 48 distinct ABC transporters have been identified in the human genome, and these can further divided into seven subfamilies based on sequence similarities. Among these transporters, the ABCB1 transporter is the most important mediator of MDR, and is responsible for chemotherapeutic drug resistance to a variety of drug, including vinca alkaloids, anthracyclines, epipodophyllotoxins and taxanes. The overexpression of ABCB1 occurs in 40– 50% of cancer patients, and is associated with a poor clinical outcome. Based on these findings, a number of studies have attempted to selectively inhibit ABCB1 activity as a strategy to reverse MDR in cancer chemotherapy. Indeed, in the past 30 years, significant efforts have been made to design and test specific ABCB1 inhibitors and this has resulted in the development of three generations of ABCB1 inhibitors. CX-4945 However, currently, none of the compounds in the three generations have been approved for clinical use. The first-generation ABCB1 inhibitors, including verapamil, quinine, and cyclosporin A lacked selectivity and produced undesirable adverse effects at plasma concentrations necessary to inhibit ABCB1. The second-generation ABCB1 inhibitors, such as valspodar/PSC-833 and biricodar/VX-710, had improved tolerability compared to the first-generation compounds. However, they produced unpredictable interactions with other transport proteins and inhibited CYP3A4, one of the major chemotherapeutic drug metabolizing enzymes, thereby reducing the the clearance and metabolism of chemotherapeutic drugs. The third-generation inhibitors were more selective for the ABCB1 transporters in ongoing clinical trials. Nonetheless, some of these compounds produced significant adverse effects and had an unfavorable pharmacokinetic profile, including poor solubility as well as reducing the clearance of clinically used anticancer drugs. Recent results from our laboratory and others indicate that several tyrosine kinase inhibitors, including imatinib, nilotinib, lapatinib, and erlotinib, can reverse MDR to antineoplastic drugs mediated by ABCtransporters. However, the reversal potential of these TKIs have not been determined in clinical trials. Consequently, it is necessary to develop more efficacious, non-toxic and less expensive compounds to reverse MDR in cancer cells. In the course of our search for compounds that reverse MDR, we found that vardenafil and tadalafil, two phosphodiesterase PF-04217903 type-5 inhibitors clinically used in the treatment of male erectile dysfunction, significantly reversed ABCB1-mediated MDR. In the present study, we conducted experiments to ascertain the reversal mechanism of vardenafil and tadalafil in ABCB1 overexpressing cancer cells. In addition, we also examined their effect on other major ABC drug transporters such as MRP1 and BCRP. One of the major mechanisms responsible to MDR in cancer cells is the overexpression of the ABCB1 transporter. However, currently, none of the ABCB1 inhibitors or modulators have been approved for clinical oncological practice. The present study demonstrates for the first time that vardenifil, a PDE-5 inhibitor used in the treatment of male erectile dysfunction, reverses ABCB1-mediated MDR in a 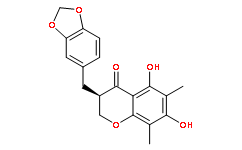 concentration-dependent manner.
concentration-dependent manner.
Considered as additional targets in the synergistic cooperation with proteasome inhibitors
Since they all are inhibited by cantharidin, and have been implicated in contributing to apoptosis regulation. The synthesis of a salubrinal-derived affinity reagent may therefore be critical to pinpoint the exact molecular target of this inhibitor and to assist in shedding further light on its mode of action. Identification of the phosphatase targeted by salubrinal 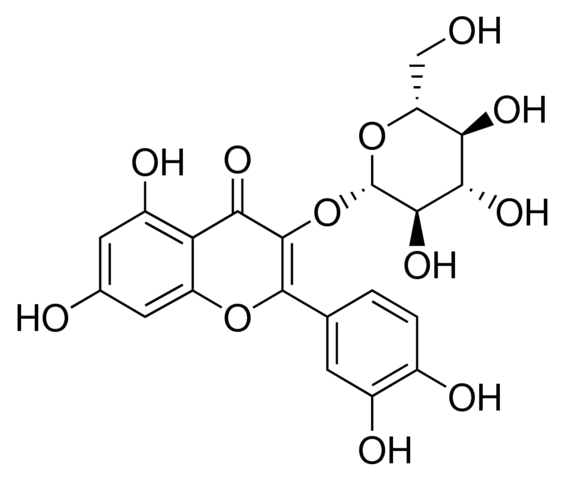 will also help to identify the corresponding phosphatase substrates and signaling pathways that are participating in survival regulation. Proteasome inhibitors exert considerable cytostatic and cytotoxic effects in particular cancer cells types already as single ICI 182780 agents, but they may be even more useful as sensitizers to apoptosis induction when delivered in combination with other anticancer drugs. Given the synergistic enhancement of proteasome inhibitor toxicity by salubrinal in K562 and other leukemic cells, salubrinal may therefore very well be added to the growing list of drugs that cooperate with proteasome inhibitor to kill hemopoietic tumor cells. It may be speculated that cancer patients receiving proteasome inhibitor treatment could benefit from the coadministration of salubrinal also for a second reason: While enhancing the killing of sensitized leukemic cells, salubrinal may at the same time ameloriate proteasome inhibitor-mediated toxicity in neuronal cells, Saveguarding neuronal cells by this means would be a desirable feature e.g. for myeloma patients receiving proteasome inhibitor treatment, since development of peripheral neuropathy is one of the major side effects and could be a direct consequence of the impairment of the ubiquitin-proteasome system. Further investigations will reveal, whether salubrinal or derivatives thereoff can be included in a therapeutic strategy that is based on the induction of ER stress and maintains a strong and selective toxicity for the tumor cells on the one hand but confers protection to neuronal and other non-transformed cells on the other. These studies will have to consider also the possibility that salubrinal may exert other side effects, due to the pleiotropic nature of phosphatase inhibitors. However, a recent proteomic study demonstrated that the number of proteins actually affected by salubrinal treatment appeared to be very limited, suggesting that salubrinal may R428 possess unique features that renders it interesting enough to further develop it into a clinically useful compound. The data presented here in summary support a paradigm shift on the protective role of the phosphatase inhibitor salubrinal during ER stress, as this compound can obviously also augment apoptosis, depending on the specific ER-stress signal and the cellular system investigated. They also suggest that the concomitant targeting of specific phosphatases in a proteasome inhibitorbased strategy to kill cancer cells could be an attractive option. The resistance of tumor cells to a variety of structurally and mechanistically unrelated cytotoxic drugs, also known as multidrug resistance, is one of the major obstacles in the successful treatment of cancer. It is estimated that approximately 500,000 new cases of cancer each year exhibit the drug resistant phenotype. One of the known causes of MDR is overexpression of the ATP-binding cassette transporters, such as P-glycoprotein, multidrug resistance proteins and breast cancer resistant protein.
will also help to identify the corresponding phosphatase substrates and signaling pathways that are participating in survival regulation. Proteasome inhibitors exert considerable cytostatic and cytotoxic effects in particular cancer cells types already as single ICI 182780 agents, but they may be even more useful as sensitizers to apoptosis induction when delivered in combination with other anticancer drugs. Given the synergistic enhancement of proteasome inhibitor toxicity by salubrinal in K562 and other leukemic cells, salubrinal may therefore very well be added to the growing list of drugs that cooperate with proteasome inhibitor to kill hemopoietic tumor cells. It may be speculated that cancer patients receiving proteasome inhibitor treatment could benefit from the coadministration of salubrinal also for a second reason: While enhancing the killing of sensitized leukemic cells, salubrinal may at the same time ameloriate proteasome inhibitor-mediated toxicity in neuronal cells, Saveguarding neuronal cells by this means would be a desirable feature e.g. for myeloma patients receiving proteasome inhibitor treatment, since development of peripheral neuropathy is one of the major side effects and could be a direct consequence of the impairment of the ubiquitin-proteasome system. Further investigations will reveal, whether salubrinal or derivatives thereoff can be included in a therapeutic strategy that is based on the induction of ER stress and maintains a strong and selective toxicity for the tumor cells on the one hand but confers protection to neuronal and other non-transformed cells on the other. These studies will have to consider also the possibility that salubrinal may exert other side effects, due to the pleiotropic nature of phosphatase inhibitors. However, a recent proteomic study demonstrated that the number of proteins actually affected by salubrinal treatment appeared to be very limited, suggesting that salubrinal may R428 possess unique features that renders it interesting enough to further develop it into a clinically useful compound. The data presented here in summary support a paradigm shift on the protective role of the phosphatase inhibitor salubrinal during ER stress, as this compound can obviously also augment apoptosis, depending on the specific ER-stress signal and the cellular system investigated. They also suggest that the concomitant targeting of specific phosphatases in a proteasome inhibitorbased strategy to kill cancer cells could be an attractive option. The resistance of tumor cells to a variety of structurally and mechanistically unrelated cytotoxic drugs, also known as multidrug resistance, is one of the major obstacles in the successful treatment of cancer. It is estimated that approximately 500,000 new cases of cancer each year exhibit the drug resistant phenotype. One of the known causes of MDR is overexpression of the ATP-binding cassette transporters, such as P-glycoprotein, multidrug resistance proteins and breast cancer resistant protein.