Epigenetic mechanisms play a dominant role in their silencing; indeed, one of the major functions of DNA methylation in mammals is to repress retroelements. The histone methyltransferase Setdb1 also plays a crucial role in their silencing. However, our understanding of the mechanism by which these various factors interact to mediate repression remains incomplete. To the best of our knowledge, this is the first study to examine the methylation state of the Avy IAP LTR in ES cells. Our initial intention was to generate a cell line that could be used in screenbased approaches to uncover novel epigenetic regulators of retrotransposon silencing. Our observation that the Avy IAP is apparently unmethylated offers insight into the unusual behaviour of the IAP at this particular locus. ES cells are derived from the inner cell mass of blastocyst embryos. Genome-wide loss of DNA methylation occurs following fertilization and prior to implantation of the blastocyst. Indeed, such hypomethylation has previously been reported at the Avy locus from blastocysts derived from both pseudoagouti and yellow animals. It is unclear whether the unmethylated state 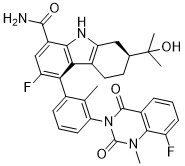 of the Avy IAP we observe in our various cell lines reflects a failure to correctly establish methylation following this erasure, or if methylation is correctly established but is rapidly lost upon ES cell derivation. Given that our bisulphite data indicate that methylation can be detected at the locus, we favor the latter interpretation that the Avy IAP is inherently epigenetically unstable during the pluripotent state. Genetic data have long supported the importance of DNA methylation in silencing IAP elements but the mechanism remains unclear. Two non-mutually exclusive models have been postulated. The first is that the presence of methylation at LTRs prevents the binding of transcriptional activators. The second is that methyl binding proteins specifically recognize methylated CpGs within LTRs and directly recruit corepressors to prevent gene expression. Our results also indicate that the prevailing model of the Avy locus requires 3,4,5-Trimethoxyphenylacetic acid refinement. That is, control of the Avy IAP LTR cryptic promoter by DNA methylation and regulation of downstream agouti expression does not operate in pluripotent stem cells. Prior studies show that in pseudoagouti animals, where the Avy IAP is presumably silent, the IAP is significantly less methylated than other closely related IAPs. Together with our observations, this indicates that the methylation state of the Avy locus does not control promoter activity of the Avy IAP. A broader implication of our study is that Mepiroxol although IAP activity is constrained by DNA methylation it is likely that not all IAP elements are dependent on this mode of regulation. It has been estimated that there are between 1,000 to 2,500 IAP elements present per haploid genome. Determining the elements controlled by DNA methylation remains technically challenging owing to the inherent repetitive nature of IAPs and unambiguously interrogating the activity of individual elements. Through interference with transcription of nearby genes, retrotransposon-derived controlling elements have the potential to affect gene expression. The mechanisms by which they are silenced are not fully understood. Our results on the well-studied Avy locus demonstrate that DNA methylation-independent mechanisms ensure repression of the locus in pluripotent stem cells. A candidate pathway involves sequence specific KRAB-ZF proteins and KAP1, the latter being important in repression of endogenous retroviruses in murine ES cells.
of the Avy IAP we observe in our various cell lines reflects a failure to correctly establish methylation following this erasure, or if methylation is correctly established but is rapidly lost upon ES cell derivation. Given that our bisulphite data indicate that methylation can be detected at the locus, we favor the latter interpretation that the Avy IAP is inherently epigenetically unstable during the pluripotent state. Genetic data have long supported the importance of DNA methylation in silencing IAP elements but the mechanism remains unclear. Two non-mutually exclusive models have been postulated. The first is that the presence of methylation at LTRs prevents the binding of transcriptional activators. The second is that methyl binding proteins specifically recognize methylated CpGs within LTRs and directly recruit corepressors to prevent gene expression. Our results also indicate that the prevailing model of the Avy locus requires 3,4,5-Trimethoxyphenylacetic acid refinement. That is, control of the Avy IAP LTR cryptic promoter by DNA methylation and regulation of downstream agouti expression does not operate in pluripotent stem cells. Prior studies show that in pseudoagouti animals, where the Avy IAP is presumably silent, the IAP is significantly less methylated than other closely related IAPs. Together with our observations, this indicates that the methylation state of the Avy locus does not control promoter activity of the Avy IAP. A broader implication of our study is that Mepiroxol although IAP activity is constrained by DNA methylation it is likely that not all IAP elements are dependent on this mode of regulation. It has been estimated that there are between 1,000 to 2,500 IAP elements present per haploid genome. Determining the elements controlled by DNA methylation remains technically challenging owing to the inherent repetitive nature of IAPs and unambiguously interrogating the activity of individual elements. Through interference with transcription of nearby genes, retrotransposon-derived controlling elements have the potential to affect gene expression. The mechanisms by which they are silenced are not fully understood. Our results on the well-studied Avy locus demonstrate that DNA methylation-independent mechanisms ensure repression of the locus in pluripotent stem cells. A candidate pathway involves sequence specific KRAB-ZF proteins and KAP1, the latter being important in repression of endogenous retroviruses in murine ES cells.
Category: GPCR Compound Library
Since IL-6 acts as a potent proinflammatory cytokine and has the ability to inhibit Treg differentiation
Liver inflammatory injury induced by IR and therefore indicated another mechanism of clinical good performance of anti-CD25 mAb in transplantations besides organ tolerance induction. Liver ischemia-reperfusion injury occurs in the clinical settings of hepatic resection surgery, hemorrhagic trauma, and liver transplantation. In the last 20 years, the rates of acute and chronic rejection have fallen dramatically, for example, the incidence of acute rejection during the first six months post-transplant has declined from over 40% in 1995 to around 15% in 2000. Part of this improvement results from increased use of selective induction agents, particularly the interleukin-2 receptor antagonists. Addition of anti-CD25 mAb to a variety of calcineurin inhibitor-based immunosuppressive regimens reduced acute rejection by 30�C50% as reported in two meta-analyses of the randomized controlled trials. These meta-analyses also demonstrated trends toward improved graft survival with anti-CD25 mAb compared with no induction. Although the protective effect for induction therapy of anti-CD25 mAb has been widely documented in liver transplantation field, the exact effect of anti-CD25 mAb on liver IR injury, however, remains poorly elucidated. The present study for the first time demonstrated that antiCD25 mAb administration shortly antecedent to liver IR induction provides protection in the initiation phase of injury. After 70% liver ischemia, anti-CD25 mAb pretreated mice displayed Ascomycin significantly preserved liver function as characterized by less histological damage and reduced serum enzymes level. We further demonstrated that the protective effect was associated with ameliorated intrahepatic inflammatory milieu and reduced CD4 + T lymphocytes as manifested by the decrease of Metyrapone proinflammatory cytokine production and the lower CD4/CD8 proportion. By employing an in vitro lymphocytes proliferation assay model, we confirmed that the protective effect of near-term antecedent anti-CD25 mAb treatment on IR-induced liver injury depend on the inhibition on the proliferation of CD4 + T lymphocytes. Taken together, our data demonstrated that near-term antecedent anti-CD25 mAb treatment provides protection for livers against subsequent IRinduced injury by inhibiting the proliferation of CD4 + T lymphocytes and mitigates intrahepatic inflammatory milieu through decreasing the proinflammatory cytokine production. Our data presented in the current report are in agreement with previous studies in the lung, kidney and brain after IR induction.  These results suggested that this brisk response, which preceded the influx of innate immune cells to the injured tissue, is mainly associated with resident T lymphocytes. These organ-resident T lymphocytes can maintain the expression of specific cytokine receptor on their cell surface and proliferate in vivo in the absence of Ag stimulation. It has been previously demonstrated that use of MHC-IIblocking antibodies has no effect on serum alanine transaminase following hepatic IR, which suggested that T cells play a role not involving the ab TCR and that lymphocyte actions occur through a non-antigenic mechanism. Treg is known as a critical role in maintaining immune homeostasis. In the model of IR injury, Treg functions to restrain excessive Teff cell responses. In line with the studies of depletion kinetics of PC61, the near-term administration of PC61 did not modify the number of Tregs significantly, and the depletion of CD4 + FoxP3 + Tregs in PC61-treated mice was already apparent 4 days after injection.
These results suggested that this brisk response, which preceded the influx of innate immune cells to the injured tissue, is mainly associated with resident T lymphocytes. These organ-resident T lymphocytes can maintain the expression of specific cytokine receptor on their cell surface and proliferate in vivo in the absence of Ag stimulation. It has been previously demonstrated that use of MHC-IIblocking antibodies has no effect on serum alanine transaminase following hepatic IR, which suggested that T cells play a role not involving the ab TCR and that lymphocyte actions occur through a non-antigenic mechanism. Treg is known as a critical role in maintaining immune homeostasis. In the model of IR injury, Treg functions to restrain excessive Teff cell responses. In line with the studies of depletion kinetics of PC61, the near-term administration of PC61 did not modify the number of Tregs significantly, and the depletion of CD4 + FoxP3 + Tregs in PC61-treated mice was already apparent 4 days after injection.
The normal variability of the human plasma proteome from healthy subjects individuals
Because of dynamic range, reproducibility and throughput limitations, 2D gel electrophoresis was surpassed by bottom-up and top-down mass spectrometry approaches for decoding protein modifications. In bottom-up approaches, proteins are digested with trypsin, and selected reaction monitoringor multiple reaction monitoringmass spectrometry is used to detect peptidesthat contain the protein modifications. Osteopontin splice variants were identified and quantified using MRM-MS ; novel proteoforms of prostate specific antigen were also identified in clinical samples via MRM-MS. In a larger population study, the concentration of three selected single amino acid polymorphism peptides, representing the Complement Component C7, Complement Factor H, and Complement Component C5 proteins, were measured by SRM-MS from 290 individuals. In another population study, serum peptide variations were studied in 500 healthy individuals using a regular MS analysis, but the peptides detected were exopeptidase products derived from relatively abundant serum proteins. Top-down MS approaches provide more accurate and complete results for protein variants identification because  there is no Cefetamet pivoxil HCl prerequisite for a priori knowledge of the protein modification in order to select the appropriate modification-specific peptide. But analyzing and quantifying intact proteins and their modifications with mass spectrometry can be challenging. Our group has devised a simple method termed mass spectrometric immunoassaythat combines a one-step affinity protein isolation with MS analysis that is ideally suited for high-throughput analysis of human plasma proteins and their variants. Antibodies are surface-immobilized in small, porous microcolumns that are fitted at the entrance of a pipettor tip. Samples are passed through the pipette tip repetitively until enough protein is bound to the antibody. Following washing with buffer and/or other mild solutions to remove non-specifically bound sample components, the proteins are eluted with a small volume of matrix solution and deposited directly onto a MALDI target for ensuing MS analysis. We’ve applied this approach to investigate the diversity of 25 human plasma proteins from a cohort of 96 healthy individuals, resulting in the detection of 76 structural variants, each occurring with different frequencies. Subsequently, we’ve screened 1,000 individuals from four geographical regions in the United States, and determined the variants and their frequencies for five proteins – beta-2-microglobulin, cystatin C, retinol binding protein, transferrin, and transthyretin. The qualitative data from those studies provided a first glimpse into the extent of protein structural diversity in the general population. Recently we have Albaspidin-AA developed and validated fully quantitative mass spectrometric immunoassays for 4 of those clinically relevant proteins – beta-2-microglobulin, cystatin C, retinol binding proteinand transthyretin. Beta-2-microglobulin is used in the diagnosis of active rheumatoid arthritis and kidney disease, and a structural variant of b2m has been associated with autoimmune disease and small-cell lung cancer. Cystatin C is a serine proteinase inhibitor with implications in renal failure. Retinol binding protein has also been implicated in renal disease, with the increased presence of its truncated variants suggested as indication of renal failure.
there is no Cefetamet pivoxil HCl prerequisite for a priori knowledge of the protein modification in order to select the appropriate modification-specific peptide. But analyzing and quantifying intact proteins and their modifications with mass spectrometry can be challenging. Our group has devised a simple method termed mass spectrometric immunoassaythat combines a one-step affinity protein isolation with MS analysis that is ideally suited for high-throughput analysis of human plasma proteins and their variants. Antibodies are surface-immobilized in small, porous microcolumns that are fitted at the entrance of a pipettor tip. Samples are passed through the pipette tip repetitively until enough protein is bound to the antibody. Following washing with buffer and/or other mild solutions to remove non-specifically bound sample components, the proteins are eluted with a small volume of matrix solution and deposited directly onto a MALDI target for ensuing MS analysis. We’ve applied this approach to investigate the diversity of 25 human plasma proteins from a cohort of 96 healthy individuals, resulting in the detection of 76 structural variants, each occurring with different frequencies. Subsequently, we’ve screened 1,000 individuals from four geographical regions in the United States, and determined the variants and their frequencies for five proteins – beta-2-microglobulin, cystatin C, retinol binding protein, transferrin, and transthyretin. The qualitative data from those studies provided a first glimpse into the extent of protein structural diversity in the general population. Recently we have Albaspidin-AA developed and validated fully quantitative mass spectrometric immunoassays for 4 of those clinically relevant proteins – beta-2-microglobulin, cystatin C, retinol binding proteinand transthyretin. Beta-2-microglobulin is used in the diagnosis of active rheumatoid arthritis and kidney disease, and a structural variant of b2m has been associated with autoimmune disease and small-cell lung cancer. Cystatin C is a serine proteinase inhibitor with implications in renal failure. Retinol binding protein has also been implicated in renal disease, with the increased presence of its truncated variants suggested as indication of renal failure.
To examine whether the L640R mutation affects the GPR56C shift to a lipid raft upon ligand stimulation
Instead of a relatively continuous and homogenous fluid of amphipathic lipids interspersed with a mosaic of proteins, it has been found that the plasma membrane contains nanoscale domains of sphingolipids, cholesterol, and membrane proteins, which together form what is referred to as ‘lipid rafts’ that function as receptor signaling platforms. Lipid rafts are resistant to cold nonionic detergent treatment, causing them to float to the top fraction of isopycnic sucrose gradients; thus they are named detergent resistant membranes. Proteins that associate with lipid rafts are defined as those that co-fractionate with DRM fractions. Therefore, cold-detergent extraction and membrane fractionation have been Salvianolic-acid-B extensively used to identify proteins associated with lipid rafts. It was recently reported that a low level of Pimozide GPR56C is constitutively associated with membrane lipid rafts. However, it is not known whether there is a dynamic presence of GPR56 in the lipid raft upon ligand stimulation. We set out to test the hypothesis that lipid raft association is required for GPR56 signaling. HEK 293T cells transfected with GPR56 cDNA were stimulated with either collagen III or acetic acid for 5 minutes. The cells were lysed in the presence of detergenton ice and subjected to DRM fractionation. GPR56N is tethered non-covalently with GPR56C on the plasma membrane, and therefore is restrictedly present in the non-raft fractions. Consistent with a previous report, we did detect a low basal level of GPR56C in the lipid raft fractions. Interestingly, we observed a significant shift of GPR56C from nonraft to lipid raft fractions upon collagen III stimulation, indicating that GPR56 probably needs lipid rafts as a platform for its signal transduction. DRM analysis was performed using this mutant receptor. Our result showed that the mutant GPR56C also translocated to lipid raft fractions after ligand stimulation, similar to the behavior of wild type GPR56. This data indicated that this disease-associated Cterminal mutation does not disrupt collagen III-induced association of GPR56 with plasma membrane lipid nanodomains. Thus, collagen III binds both wild type and the L640R mutant, resulting in the C-terminal fragment associating with DRMs. We previously showed that Collagen III is a ligand of GPR56 in the developing brain. Upon binding to collagen III, GPR56 activates RhoA via coupling to Ga12/13. Here, we discover that collagen III binding also induces release of the GPR56N fragment, allowing the GPR56C fragment to associate with DRMs. Surprisingly, the L640R mutation does not inhibit these processes, but instead blocks downstream RhoA activation. Like most other adhesion GPCRs, GPR56 is autocatalytically cleaved through the GPS motif between amino acids histidine-381 and leucine-382 into N- and C-terminal fragments, GPR56N and GPR56C, respectively. Although mutations in the GPS domain disrupt this cleavage 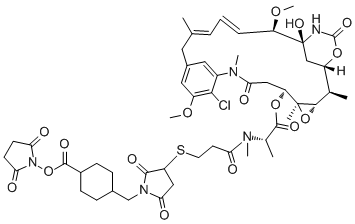 and cause human BFPP disease, the biological significance of this cleavage is not entirely clear. We previously showed that the cleaved GPR56N remains associated with GPR56C at the plasma membrane. Furthermore, work from Hall’s group showed that overexpression of GPR56C alone results in constitutive activation of RhoA. We therefore hypothesized that the association of GPR56N and GPR56C keeps the receptor in an inactivated state, and the binding of collagen III activates the receptor by removing GPR56N from GPR56C.
and cause human BFPP disease, the biological significance of this cleavage is not entirely clear. We previously showed that the cleaved GPR56N remains associated with GPR56C at the plasma membrane. Furthermore, work from Hall’s group showed that overexpression of GPR56C alone results in constitutive activation of RhoA. We therefore hypothesized that the association of GPR56N and GPR56C keeps the receptor in an inactivated state, and the binding of collagen III activates the receptor by removing GPR56N from GPR56C.
Resistant individuals compared to insulin-sensitive individuals, we failed to detect PTPRT protein in mouse adipose
Neither could we detect PTPRT protein in liver or muscle. Our data indicate that PTPRT does not directly modulate insulin sensitivity in peripheral tissues. Instead, PTPRT may indirectly impact peripheral insulin resistance through affecting the nervous Gambogic-acid system control of energy homeostasis. Fibroblast growth factorsfunction in numerous processes throughout 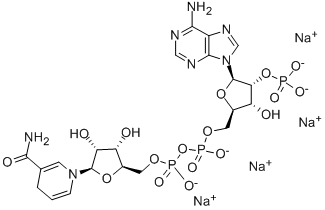 embryonic development, such as the induction and patterning of germ cell layers, body axis formation and organogenesis. Out of 22 human and mouse FGFs, 18 bind to a distinct set of cell-surface FGF receptorsto initiate intracellular signalling that results in cellular responses, including cell proliferation and differentiation. Our understanding of the physiological roles of FGF ligands and their receptors has been helped enormously by the study of mouse knockouts. Ranging from early embryonic lethality to adult metabolic abnormalities, these mutant phenotypes reveal the breadth of impact of FGF signalling. Testis determination in the embryo normally requires the Ylinked gene Sry to initiate the commitment of somatic cells in the developing bipotential gonad to the Sertoli cell fate. SRY effects this commitment through its positive effects on the expression of Sox9, a gene that is itself necessary and sufficient for testis development. The analysis of mice lacking FGF9 first revealed a role for this signalling pathway in testis determination. Fgf9-deficient animals die around birth due to severe lung hypoplasia and, on a mixed genetic background, XY embryos exhibit a range of gonadal abnormalities ranging from testicular hypoplasia to complete sex reversal. On the C57BL/6Jbackground, which is sensitised to disruptions to testis determination, XY Fgf9-deficient embryos consistently exhibit gonadal sex reversal, indicating that some of the earliest processes in testis determination are disrupted by the absence of FGF9. Subsequent studies revealed an important role for FGF9 in maintaining high levels of Sox9 expression in the developing XY gonad, mediated at least partly by its inhibitory effects on ovarydetermining genes such as Wnt4. It has also been proposed that the rapid diffusion of secreted FGF9 along the long, thin gonad at around 11.5 dpc prevents any appreciable delay in the gonadal poles receiving the masculinising signal begun by expression of SRY at the centre of the gonad. Any such delay may result in ovotestis or ovary Ursolic-acid development in an XY embryo due to the restricted time window that is thought to define the competence of cells to respond to SRY and its downstream effectors. FGF9 acts as a paracrine FGF, mediating its effects locally by binding to and activating one of four tyrosine kinase FGFRs, using heparin sulphate proteoglycancofactor-association as a means of regulating ligand distribution and receptor binding. Loss-of-function genetic studies have identified FGFR2 as the likely receptor for embryonic gonadal FGF9. Embryos lacking FGFR2 die mid-gestation, at around 10.5 dpc, precluding a study of the effects of this loss on testis determination. Conditional gene targeting revealed partial XY gonadal sex reversal when Fgfr2 deletion was restricted temporally, from around 10.5 dpc onwards, or spatially, to gonadal somatic cells. Here we report the identification, in a mouse forward genetic screen, of a novel sex-reversing mutant allele of Fgfr2. Previous studies of Fgfr2 function in testis determination have relied on conditional gene targeting.
embryonic development, such as the induction and patterning of germ cell layers, body axis formation and organogenesis. Out of 22 human and mouse FGFs, 18 bind to a distinct set of cell-surface FGF receptorsto initiate intracellular signalling that results in cellular responses, including cell proliferation and differentiation. Our understanding of the physiological roles of FGF ligands and their receptors has been helped enormously by the study of mouse knockouts. Ranging from early embryonic lethality to adult metabolic abnormalities, these mutant phenotypes reveal the breadth of impact of FGF signalling. Testis determination in the embryo normally requires the Ylinked gene Sry to initiate the commitment of somatic cells in the developing bipotential gonad to the Sertoli cell fate. SRY effects this commitment through its positive effects on the expression of Sox9, a gene that is itself necessary and sufficient for testis development. The analysis of mice lacking FGF9 first revealed a role for this signalling pathway in testis determination. Fgf9-deficient animals die around birth due to severe lung hypoplasia and, on a mixed genetic background, XY embryos exhibit a range of gonadal abnormalities ranging from testicular hypoplasia to complete sex reversal. On the C57BL/6Jbackground, which is sensitised to disruptions to testis determination, XY Fgf9-deficient embryos consistently exhibit gonadal sex reversal, indicating that some of the earliest processes in testis determination are disrupted by the absence of FGF9. Subsequent studies revealed an important role for FGF9 in maintaining high levels of Sox9 expression in the developing XY gonad, mediated at least partly by its inhibitory effects on ovarydetermining genes such as Wnt4. It has also been proposed that the rapid diffusion of secreted FGF9 along the long, thin gonad at around 11.5 dpc prevents any appreciable delay in the gonadal poles receiving the masculinising signal begun by expression of SRY at the centre of the gonad. Any such delay may result in ovotestis or ovary Ursolic-acid development in an XY embryo due to the restricted time window that is thought to define the competence of cells to respond to SRY and its downstream effectors. FGF9 acts as a paracrine FGF, mediating its effects locally by binding to and activating one of four tyrosine kinase FGFRs, using heparin sulphate proteoglycancofactor-association as a means of regulating ligand distribution and receptor binding. Loss-of-function genetic studies have identified FGFR2 as the likely receptor for embryonic gonadal FGF9. Embryos lacking FGFR2 die mid-gestation, at around 10.5 dpc, precluding a study of the effects of this loss on testis determination. Conditional gene targeting revealed partial XY gonadal sex reversal when Fgfr2 deletion was restricted temporally, from around 10.5 dpc onwards, or spatially, to gonadal somatic cells. Here we report the identification, in a mouse forward genetic screen, of a novel sex-reversing mutant allele of Fgfr2. Previous studies of Fgfr2 function in testis determination have relied on conditional gene targeting.