Since they all are inhibited by cantharidin, and have been implicated in contributing to apoptosis regulation. The synthesis of a salubrinal-derived affinity reagent may therefore be critical to pinpoint the exact molecular target of this inhibitor and to assist in shedding further light on its mode of action. Identification of the phosphatase targeted by salubrinal 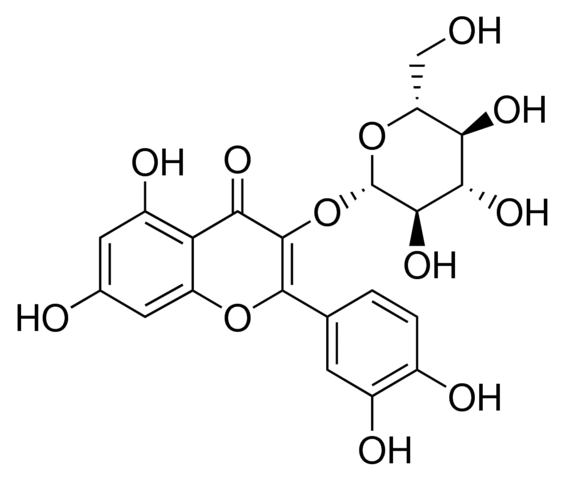 will also help to identify the corresponding phosphatase substrates and signaling pathways that are participating in survival regulation. Proteasome inhibitors exert considerable cytostatic and cytotoxic effects in particular cancer cells types already as single ICI 182780 agents, but they may be even more useful as sensitizers to apoptosis induction when delivered in combination with other anticancer drugs. Given the synergistic enhancement of proteasome inhibitor toxicity by salubrinal in K562 and other leukemic cells, salubrinal may therefore very well be added to the growing list of drugs that cooperate with proteasome inhibitor to kill hemopoietic tumor cells. It may be speculated that cancer patients receiving proteasome inhibitor treatment could benefit from the coadministration of salubrinal also for a second reason: While enhancing the killing of sensitized leukemic cells, salubrinal may at the same time ameloriate proteasome inhibitor-mediated toxicity in neuronal cells, Saveguarding neuronal cells by this means would be a desirable feature e.g. for myeloma patients receiving proteasome inhibitor treatment, since development of peripheral neuropathy is one of the major side effects and could be a direct consequence of the impairment of the ubiquitin-proteasome system. Further investigations will reveal, whether salubrinal or derivatives thereoff can be included in a therapeutic strategy that is based on the induction of ER stress and maintains a strong and selective toxicity for the tumor cells on the one hand but confers protection to neuronal and other non-transformed cells on the other. These studies will have to consider also the possibility that salubrinal may exert other side effects, due to the pleiotropic nature of phosphatase inhibitors. However, a recent proteomic study demonstrated that the number of proteins actually affected by salubrinal treatment appeared to be very limited, suggesting that salubrinal may R428 possess unique features that renders it interesting enough to further develop it into a clinically useful compound. The data presented here in summary support a paradigm shift on the protective role of the phosphatase inhibitor salubrinal during ER stress, as this compound can obviously also augment apoptosis, depending on the specific ER-stress signal and the cellular system investigated. They also suggest that the concomitant targeting of specific phosphatases in a proteasome inhibitorbased strategy to kill cancer cells could be an attractive option. The resistance of tumor cells to a variety of structurally and mechanistically unrelated cytotoxic drugs, also known as multidrug resistance, is one of the major obstacles in the successful treatment of cancer. It is estimated that approximately 500,000 new cases of cancer each year exhibit the drug resistant phenotype. One of the known causes of MDR is overexpression of the ATP-binding cassette transporters, such as P-glycoprotein, multidrug resistance proteins and breast cancer resistant protein.
will also help to identify the corresponding phosphatase substrates and signaling pathways that are participating in survival regulation. Proteasome inhibitors exert considerable cytostatic and cytotoxic effects in particular cancer cells types already as single ICI 182780 agents, but they may be even more useful as sensitizers to apoptosis induction when delivered in combination with other anticancer drugs. Given the synergistic enhancement of proteasome inhibitor toxicity by salubrinal in K562 and other leukemic cells, salubrinal may therefore very well be added to the growing list of drugs that cooperate with proteasome inhibitor to kill hemopoietic tumor cells. It may be speculated that cancer patients receiving proteasome inhibitor treatment could benefit from the coadministration of salubrinal also for a second reason: While enhancing the killing of sensitized leukemic cells, salubrinal may at the same time ameloriate proteasome inhibitor-mediated toxicity in neuronal cells, Saveguarding neuronal cells by this means would be a desirable feature e.g. for myeloma patients receiving proteasome inhibitor treatment, since development of peripheral neuropathy is one of the major side effects and could be a direct consequence of the impairment of the ubiquitin-proteasome system. Further investigations will reveal, whether salubrinal or derivatives thereoff can be included in a therapeutic strategy that is based on the induction of ER stress and maintains a strong and selective toxicity for the tumor cells on the one hand but confers protection to neuronal and other non-transformed cells on the other. These studies will have to consider also the possibility that salubrinal may exert other side effects, due to the pleiotropic nature of phosphatase inhibitors. However, a recent proteomic study demonstrated that the number of proteins actually affected by salubrinal treatment appeared to be very limited, suggesting that salubrinal may R428 possess unique features that renders it interesting enough to further develop it into a clinically useful compound. The data presented here in summary support a paradigm shift on the protective role of the phosphatase inhibitor salubrinal during ER stress, as this compound can obviously also augment apoptosis, depending on the specific ER-stress signal and the cellular system investigated. They also suggest that the concomitant targeting of specific phosphatases in a proteasome inhibitorbased strategy to kill cancer cells could be an attractive option. The resistance of tumor cells to a variety of structurally and mechanistically unrelated cytotoxic drugs, also known as multidrug resistance, is one of the major obstacles in the successful treatment of cancer. It is estimated that approximately 500,000 new cases of cancer each year exhibit the drug resistant phenotype. One of the known causes of MDR is overexpression of the ATP-binding cassette transporters, such as P-glycoprotein, multidrug resistance proteins and breast cancer resistant protein.