Which are FDA-approved agents, and 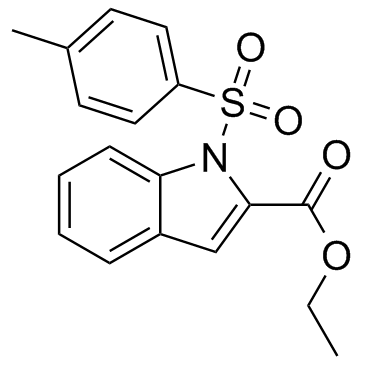 the non-FDA-labeled PPI tenatoprazole. The pharmacophore models described for OCT1 and OCT2 share a hydrophobic interaction site and a positive ionizable site. The pharmacophore models of the present study are in line with these models in having at least 1 hydrophobic interaction site as well. The lack of a positive ionizable site in our models is probably due to the fact that many of the compounds selected for the training sets are neutral at pH 7.4. Our pharmacophore models predict PPIs to be very potent inhibitors of OCT1, OCT2, and OCT3, mainly due to their hydrophobic features and presence of H-bond acceptor sites. In order to validate the data of the in silico pharmacophore modeling, we generated cell systems stably expressing recombinant human OCT1, OCT2, or OCT3. All 3 transfected HEK cell lines BIBW2992 expressed functionally active organic cation transporters as demonstrated by time-dependent TEA and metformin uptake, which are both well-established substrates of OCTs. Consistent with these functional data, the recombinant OCT proteins were detected in the plasma membrane of the OCT-expressing HEK cells as well as in membrane fractions from these cells as expected. The most striking result of our study was a potent inhibition of metformin uptake transport by all five PPIs for all 3 OCT proteins tested with IC50 values in the low micromolar range, similar to calculated total PPI concentrations in portal venous blood. Moreover, we could clearly show that none of these PPIs are substrates for the 3 OCT transport proteins. The fact that drugs are potent OCT inhibitors without being substrates, is in agreement with results obtained for several other compounds. Moreover, OCT1- and OCT3-mediated metformin uptake appears to be activated by low concentrations of selected PPIs, which is in line with previous observations reported for carvedilol and OCT2-mediated metformin uptake but also for other uptake transporters and inhibitors. However, underlying molecular mechanisms are currently unknown. Given the role of OCT1 for metformin action and of OCT2 for renal secretion of metformin, efforts have been made to identify physicochemical parameters that may predict whether a compound inhibits the OCT transporters. One study showed that a positive charge at pH 7.4 and a high lipophilicity are the main properties of potent OCT1 inhibitors. The PLS analysis revealed that the ClogP value likewise appears to be a relevant factor for Rapamycin explaining OCT1 inhibition by the 5 PPIs. For OCT2, one study also identified the ClogP value as a principal factor for potent inhibition, while in another study the TPSA value was predictive for inhibition. However, neither the ClogP value nor the TPSA value are apparently predictive for OCT2 or OCT3 inhibition by PPIs. It therefore remains unclear which physicochemical parameters determine the inhibition potency of PPIs towards OCT2 and OCT3. Another physicochemical parameter, i.e. the charge at pH 7.4 that was identified as a relevant property of OCT1 inhibitors, is apparently not sufficient for predicting a compound��s inhibition potencytowardsOCTs since PPIs are neutral at pH 7.4 and it has been shown that several other OCT inhibitors are likewise not positively charged. Currently, to the best of our knowledge no interaction studies in healthy volunteers and/or patients exist elucidating pharmacokinetic and/or �Cdynamic consequences of a combined therapy of metformin and PPIs.
the non-FDA-labeled PPI tenatoprazole. The pharmacophore models described for OCT1 and OCT2 share a hydrophobic interaction site and a positive ionizable site. The pharmacophore models of the present study are in line with these models in having at least 1 hydrophobic interaction site as well. The lack of a positive ionizable site in our models is probably due to the fact that many of the compounds selected for the training sets are neutral at pH 7.4. Our pharmacophore models predict PPIs to be very potent inhibitors of OCT1, OCT2, and OCT3, mainly due to their hydrophobic features and presence of H-bond acceptor sites. In order to validate the data of the in silico pharmacophore modeling, we generated cell systems stably expressing recombinant human OCT1, OCT2, or OCT3. All 3 transfected HEK cell lines BIBW2992 expressed functionally active organic cation transporters as demonstrated by time-dependent TEA and metformin uptake, which are both well-established substrates of OCTs. Consistent with these functional data, the recombinant OCT proteins were detected in the plasma membrane of the OCT-expressing HEK cells as well as in membrane fractions from these cells as expected. The most striking result of our study was a potent inhibition of metformin uptake transport by all five PPIs for all 3 OCT proteins tested with IC50 values in the low micromolar range, similar to calculated total PPI concentrations in portal venous blood. Moreover, we could clearly show that none of these PPIs are substrates for the 3 OCT transport proteins. The fact that drugs are potent OCT inhibitors without being substrates, is in agreement with results obtained for several other compounds. Moreover, OCT1- and OCT3-mediated metformin uptake appears to be activated by low concentrations of selected PPIs, which is in line with previous observations reported for carvedilol and OCT2-mediated metformin uptake but also for other uptake transporters and inhibitors. However, underlying molecular mechanisms are currently unknown. Given the role of OCT1 for metformin action and of OCT2 for renal secretion of metformin, efforts have been made to identify physicochemical parameters that may predict whether a compound inhibits the OCT transporters. One study showed that a positive charge at pH 7.4 and a high lipophilicity are the main properties of potent OCT1 inhibitors. The PLS analysis revealed that the ClogP value likewise appears to be a relevant factor for Rapamycin explaining OCT1 inhibition by the 5 PPIs. For OCT2, one study also identified the ClogP value as a principal factor for potent inhibition, while in another study the TPSA value was predictive for inhibition. However, neither the ClogP value nor the TPSA value are apparently predictive for OCT2 or OCT3 inhibition by PPIs. It therefore remains unclear which physicochemical parameters determine the inhibition potency of PPIs towards OCT2 and OCT3. Another physicochemical parameter, i.e. the charge at pH 7.4 that was identified as a relevant property of OCT1 inhibitors, is apparently not sufficient for predicting a compound��s inhibition potencytowardsOCTs since PPIs are neutral at pH 7.4 and it has been shown that several other OCT inhibitors are likewise not positively charged. Currently, to the best of our knowledge no interaction studies in healthy volunteers and/or patients exist elucidating pharmacokinetic and/or �Cdynamic consequences of a combined therapy of metformin and PPIs.