The infection by root pathogenic oomycetes is initiated by the release from sporangia of motile, biflagellate and wall-less zoospores, which encyst and adhere to the host surface following a chemotactically and electrotactically swimming stage. The cysts germinate by forming the germ tubes and start to penetrate the plant cuticle directly with the aid of secreted enzymes and colonize host tissues. Previous studies have shown that these pre-infection structures are rich in molecules involved in establishment of infection and elicitation of plant defenses. The unraveling of the molecular processes regulating the life cycle of Phytophthora is therefore important to identify determinants of pathogenesis and develop appropriate control strategies. The heterothallic oomycete Phytophthora capsici Leonian can cause seed, root, crown, foliar and fruit rot on a number of important crops, such as solanaceous crops, cucurbits and bean crops. P. capsici is also recorded as a pathogen of Allium cepa, Nicotiana benthamiana and Arabidopsis thaliana. To date, the diseases caused by P. capsici have become devastating ones of global economic importance. The cost that P. capsici inflicts upon worldwide vegetable production is estimated to be $1 billion every year. Unfortunately, the control of P. capsici is a difficult task as there has been no available effective chemical or cultural strategy. In order to improve methods for controlling Phytophthora diseases, it is essential to understand the molecular mechanisms by which the pathogen suppresses or escapes the host plant defenses. Recent studies on plant pathogenic oomycetes have demonstrated that these pathogens accomplish their penetration and colonization of host plants by manipulating their hosts through a diverse arsenal of secreted proteins. According to their potential targeting sites in the host plant, these secreted effector proteins are Mepiroxol classified into two classes, apoplastic and cytoplasmic effectors. Apoplastic effectors, including elicitins, PcF/SCR-like proteins and NLPs, are located at the interface between pathogen and its host and fulfill a function on the outside of the host cell. Elicitins, one type of pathogen associated molecular patterns, can trigger plant cell death response, normally known as hypersensitive reaction that is characteristic of plant defense response. These proteins share a conserved 98-amino-acid domain with a characteristic signature of six cysteine residues that form three distinct disulfide bonds. Members of the PcF/SCR toxin family, small cysteine-rich proteins, are thought to be involved in the induction of plant cell death. Other toxins secreted by oomycetes belong to NLPs that elicit plant cell death in dicotyledonous plants. In contrast, 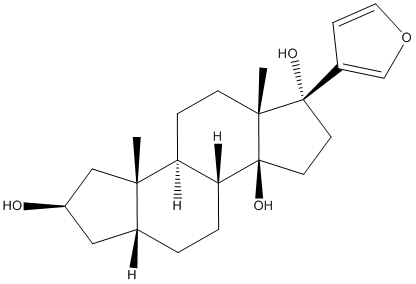 cytoplasmic effectors are able to translocate inside host cells where they interfere with the host defense responses. Two important groups of translocated effectors are RXLR and CRN protein families. RXLR effectors are named after an N-terminal RXLR amino acid motif contained by the first characterized effectors of this class, where ��X’denotes a nonconserved amino acid residue. This motif assists in the translocation of the proteins into the Alprostadil host’s cytoplasm where the effectors function as virulence or avirulence factors depending on the host genotype. Host translocation may also occur with variations of the RXLR motif or even in the absence of any such motif may. A structural dissection of these proteins has revealed a signal peptide followed by an RXLR motif at the N-terminus, which are required for secretion and targeting of the proteins whereas the C-terminus executes the actual effector activity.
cytoplasmic effectors are able to translocate inside host cells where they interfere with the host defense responses. Two important groups of translocated effectors are RXLR and CRN protein families. RXLR effectors are named after an N-terminal RXLR amino acid motif contained by the first characterized effectors of this class, where ��X’denotes a nonconserved amino acid residue. This motif assists in the translocation of the proteins into the Alprostadil host’s cytoplasm where the effectors function as virulence or avirulence factors depending on the host genotype. Host translocation may also occur with variations of the RXLR motif or even in the absence of any such motif may. A structural dissection of these proteins has revealed a signal peptide followed by an RXLR motif at the N-terminus, which are required for secretion and targeting of the proteins whereas the C-terminus executes the actual effector activity.
Month: May 2019
Increased metabolism in co-cultures modeling infectious disease can drastically alter the media composition
Long-term culturing of Giardia trophozoites with human epithelial cells in vitro is challenging due to the microaerophilic nature of the parasite. To overcome the parasite survival barrier, most in vitro studies have utilized high multiplicities of infection in addition to the short incubation times. These conditions do not reflect a typical Giardia infection where a low infectious dose leads to an active infection that can span several days to weeks. Therefore, establishing an in vitro model that allows for the protracted coculture of host epithelial and immune cells with Giardia trophozoites would greatly contribute to the understanding of late infection interactions. Our model utilizes transwell inserts to co-culture a human intestinal epithelial cell line and a 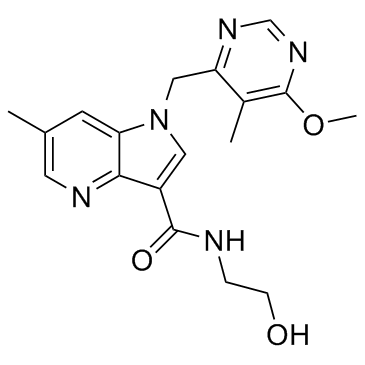 murine peritoneal macrophage cell line in a manner that represents the apical-basolateral orientation of the small intestine. IC21 murine peritoneal macrophages were selected for their similarity to human macrophages, including their typical macrophage morphology in culture, their expression of macrophagespecific antigens, their activation by lipopolysaccharide, their phagocytic ability, and their expression of IgG receptors. Caco-2 cells, derived from a human colonic adenocarcinoma retain both morphologic and phenotypic characteristics of intestinal epithelial cells when fully differentiated, including polarized morphology, microvilli on the apical surface, expression of brush border enzymes, and adjacent cell tight junctions. The in vitro Albaspidin-AA differentiation of Caco-2 cells into a phenotype similar to small intestinal epithelial cells is a timemediated event that is Alprostadil dependent on many factors, including passage number, seeding density, media composition, and substrate support. In our study, the use of Caco-2 cells at three days post plating allows for the assessment of how the parasite affects the proliferation and differentiation process of enterocytes in the intestine. The epithelial barrier of the intestine is replenished every 4�C5 days; therefore, enterocyte renewal through stem cell differentiation is critical for normal functioning of the human gut. Caco-2 cells have been used to model the differentiation process of enterocytes in the small intestine. Although immature proliferating Caco2 cells show differences in gene expression, the protein expression profiles are remarkably similar when compared to fully differentiated Caco-2 cells. Thus far, Caco-2 cells are the best described enterocyte cell line and the most common epithelial cell line used in in vitro Giardia�Chost interactions. Therefore, using Caco-2 cells to characterize our co-culture model allows us to compare our results with those published in the literature. Following establishment of cell-cell communication and epithelial monolayer formation, Giardia trophozoites were added to the system using a lower starting density than what has previously been reported. This model allows the parasite to proliferate in culture, more accurately reflects the infection in vivo, and allows for the characterization of host-Giardia interactions from the start of an infection through its termination, including the role of immune cells in limiting the infection. Our data indicate that both parasites and epithelial cells are viable in the 90% DMEM/10% Giardia media mixture. Using transwell inserts filled with medium, we were able to limit the oxygen exposure of the parasites, while allowing the epithelial cells to exchange oxygen and nutrients through their basolateral surface. Giardia proliferates in the 90% DMEM/10% Giardia media and saturates the insert surface at 5 days post-infection. The drop in parasite density after 5 days is attributed to daily feeding of the insert cultures.
murine peritoneal macrophage cell line in a manner that represents the apical-basolateral orientation of the small intestine. IC21 murine peritoneal macrophages were selected for their similarity to human macrophages, including their typical macrophage morphology in culture, their expression of macrophagespecific antigens, their activation by lipopolysaccharide, their phagocytic ability, and their expression of IgG receptors. Caco-2 cells, derived from a human colonic adenocarcinoma retain both morphologic and phenotypic characteristics of intestinal epithelial cells when fully differentiated, including polarized morphology, microvilli on the apical surface, expression of brush border enzymes, and adjacent cell tight junctions. The in vitro Albaspidin-AA differentiation of Caco-2 cells into a phenotype similar to small intestinal epithelial cells is a timemediated event that is Alprostadil dependent on many factors, including passage number, seeding density, media composition, and substrate support. In our study, the use of Caco-2 cells at three days post plating allows for the assessment of how the parasite affects the proliferation and differentiation process of enterocytes in the intestine. The epithelial barrier of the intestine is replenished every 4�C5 days; therefore, enterocyte renewal through stem cell differentiation is critical for normal functioning of the human gut. Caco-2 cells have been used to model the differentiation process of enterocytes in the small intestine. Although immature proliferating Caco2 cells show differences in gene expression, the protein expression profiles are remarkably similar when compared to fully differentiated Caco-2 cells. Thus far, Caco-2 cells are the best described enterocyte cell line and the most common epithelial cell line used in in vitro Giardia�Chost interactions. Therefore, using Caco-2 cells to characterize our co-culture model allows us to compare our results with those published in the literature. Following establishment of cell-cell communication and epithelial monolayer formation, Giardia trophozoites were added to the system using a lower starting density than what has previously been reported. This model allows the parasite to proliferate in culture, more accurately reflects the infection in vivo, and allows for the characterization of host-Giardia interactions from the start of an infection through its termination, including the role of immune cells in limiting the infection. Our data indicate that both parasites and epithelial cells are viable in the 90% DMEM/10% Giardia media mixture. Using transwell inserts filled with medium, we were able to limit the oxygen exposure of the parasites, while allowing the epithelial cells to exchange oxygen and nutrients through their basolateral surface. Giardia proliferates in the 90% DMEM/10% Giardia media and saturates the insert surface at 5 days post-infection. The drop in parasite density after 5 days is attributed to daily feeding of the insert cultures.
Proximal deletion of CFA27 was confirmed by ddPCR and found in tumor genomes
To assess apoptosis in our model, we compared caspase-3 activation in Caco-2 cells on inserts to the long-established monoculture plate environment. Our results indicate that live WBC6 trophozoites can induce apoptosis in a time-dependent manner. Since sonicated Giardia WBC6 parasites fail to produce the same response, this may indicate this particular Giardia strain mediates host cell death through a direct parasite-epithelial cell interaction. The difference in apoptosis observed between plate and insert cultures at 5 days in our studies is likely due to parasite density in the different culture conditions. Significantly more parasites are observed in the insert environment even though the plate and insert cultures received the same starting density of parasites. These findings suggest that nucleotide divergence does not influence cleavage precision, but that nucleotide bias in the 59 and 39 ends of the loop potentially influences miRNA maturation. Herein, canonical miRNA loop Diperodon sequences were collected, with variations in the loops potentially based on the phenomenon of multiple isomiRs, with the canonical miRNA sequences not necessarily the most dominant sequence. However, according to length distributions and terminal end nucleotide compositions, it can be concluded that loop sequences tend to be longer in higher animal species, with rapid evolution of the loop sequences further driving miRNA gene evolution. Stable isomiR expression profiles indicate relative cleavage, which may be closely related to the dominant nucleotide distributions. This study further enriches the understanding of miRNA biogenesis as it relates to loop sequences across different animal species and among homologous miRNAs, particularly considering the phenomenon of multiple isomiRs. Proteins destined for the secretory pathway are inserted into the ER cotranslationally and subjected to quality control. ER molecular chaperones and folding enzymes such as BiP, calnexin, and protein disulfide isomerase facilitate the correct folding or degradation of these newly synthesized proteins as well as of misfolded proteins. The accumulation of misfolded proteins in the ER beyond the capacity of quality control causes ER stress and induces the unfolded protein response. Further ER stress can cause cellular dysfunction and cell death, resulting in diverse human disorders such as neurodegenerative diseases. Mammalian ER luminal chaperones have a carboxyl terminal Lys-Asp-Glu-Leu amino acid sequence, which is recognized by the KDEL receptor in post-ER compartments. ER chaperones and the KDEL receptor are sorted into the transport vesicles coated with coat protein I complex and retrieved to the ER. Yeast BiP is essential for survival, while the deletion of the retrieval sequence is dispensable because the UPR is activated and the loss of the chaperone in the ER is compensated for. The complete depletion of BiP also has lethal effects on mammalian early embryonic cells. In order to elucidate the physiological processes that are sensitive to the retrieval of BiP during development and adulthood in multi-cellular organisms, we previously produced knock-in mice expressing a D-Pantothenic acid sodium mutant BiP in which the retrieval sequence was deleted by homologous recombination. The homozygous mutant BiP mice died within several hours after birth due to respiratory failure with impaired biosynthesis of the pulmonary surfactant.
A transcriptional elicitins was high at the GC stage and another peak later in infection stage on soybean
These data suggest that P. capsici expresses some genes in common with other Phytophthora pathogens, but the timing may differ. Our RXLR dataset includes several predicted orthologs to known avirulence genes such as P. sojae Avr1b and P. infestans Avr3a. For instance, the predicted protein Pc22053 showed 33% identity with P. sojae Avr1b. Epimedoside-A However, Pc22053 can not cause cell death in N. benthamiana during the transient expression assays. This is not surprising as data from P. sojae indicate that Avr1b induced cell death occurs only on soybean plants carrying Rps1b. To explore this further, we tested for transcripts of Pc22053 by RT-PCR and observed its highly expression during the host infection. Furthermore, we observed that Pc22053 can suppress the HR induced by all 8 different effectors. Similarly, selected RXLR effectors and the RXLR-like SNE1 protein from other Phytophthora species have demonstrated their ability to suppress cell death and defense. We demonstrated that one P. capsici CRN gene Pc506611 does not cause plant cell death when transiently expressed in N. benthamiana. This is not a rare case because recent studies show that cell death induction is not a universal feature of CRN proteins. Over-expression of Phytophthora CRN domains only induce necrosis in a few cases. Intriguingly, when the putative Pc506611 CRN protein sequence was compared against NCBI Nr database, a ubiquitin-like domain was detected in this protein. The family of proteins containing UBL and ubiquitin-associated domains has been implicated in proteasomal degradation. Whether the protein contributed to the pathogenicity through ubiquitin pathway during the host-oomycete interaction is yet to be 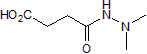 determined. Furthermore, the high expression of Pc506611 during the GC and early infection stages leads us to suspect that it contributes positively to the pathogen’s virulence. Ectopic expression showed that this gene can suppress plant cell death caused by all tested cell death inducers but R3a/Avr3a. Data from a recent study on P. sojae CRNs indicate that one CRN, PsCRN115, also suppresses cell death elicited by e.g. PsojNIP and PsCRN63. The similar results indicated that this family of effectors also has similar abilities to RXLR effectors in suppressing plant defense. Host cell death or the HR is an effective and ultimate defense mechanism against obligate biotrophic and hemibiotrophic pathogens. However, delayed HR could be counterproductive and benefit hemibiotrophic pathogens in necrotrophic growth stages. P. capsici is a hemibiotroph and switches from biotrophic to necrotrophic growth 18 to 42 h following the invasion of N. benthamiana leaves. Therefore, the ability of P. capsici to suppress or delay the HR of host tissue is likely a major component of its D-Pantothenic acid sodium pathogenic strategy, as is for P. sojae. We observed a small number of NLPs and elicitins with differential expression. DEG analysis showed that three NLPs showed increased expression at GC compared with MY. RT-PCR analysis demonstrated that they are highly expressed during the infection stages. Similarly, a study on P. sojae NLPs found that most NLP are highly expressed during cyst germination and infection stages. Ectopic expression showed that one of our P. capsici NLP genes triggered cell death in N. benthamiana. Although it is accepted that NLPs can contribute to virulence as toxins, it has been reported that many members of this family do not possess obvious toxic activity.
determined. Furthermore, the high expression of Pc506611 during the GC and early infection stages leads us to suspect that it contributes positively to the pathogen’s virulence. Ectopic expression showed that this gene can suppress plant cell death caused by all tested cell death inducers but R3a/Avr3a. Data from a recent study on P. sojae CRNs indicate that one CRN, PsCRN115, also suppresses cell death elicited by e.g. PsojNIP and PsCRN63. The similar results indicated that this family of effectors also has similar abilities to RXLR effectors in suppressing plant defense. Host cell death or the HR is an effective and ultimate defense mechanism against obligate biotrophic and hemibiotrophic pathogens. However, delayed HR could be counterproductive and benefit hemibiotrophic pathogens in necrotrophic growth stages. P. capsici is a hemibiotroph and switches from biotrophic to necrotrophic growth 18 to 42 h following the invasion of N. benthamiana leaves. Therefore, the ability of P. capsici to suppress or delay the HR of host tissue is likely a major component of its D-Pantothenic acid sodium pathogenic strategy, as is for P. sojae. We observed a small number of NLPs and elicitins with differential expression. DEG analysis showed that three NLPs showed increased expression at GC compared with MY. RT-PCR analysis demonstrated that they are highly expressed during the infection stages. Similarly, a study on P. sojae NLPs found that most NLP are highly expressed during cyst germination and infection stages. Ectopic expression showed that one of our P. capsici NLP genes triggered cell death in N. benthamiana. Although it is accepted that NLPs can contribute to virulence as toxins, it has been reported that many members of this family do not possess obvious toxic activity.
In macrophages during intracellular infection and required for the maturation of the BCV into ER-derived replicative organelles
A functional T4SS which includes the translocation of secreted effector proteins into the host cell or the vacuolar membrane is activated, thereby promoting Brucella’s survival and replication. The expression of the T4SS VirB is tightly regulated both in vitro and in vivo by several molecular systems. Direct interactions with the promoter region of VirB has been demonstrated for integration host factor, a DNAbinding and -bending protein with roles in local DNA structural organization and transcriptional regulation of a wide variety of bacterial genes, VjbR the quorum sensing-dependent regulator involved in surface modifications of Brucella, HutC, the transcriptional repressor of the histidine genes, and the two-component regulatory system BvrR/BvrS that participates in the homeostasis of the outer membrane controlling the Diatrizoic acid structure of the lipopolysaccharide, the expression of periplasmic and outer membrane proteins and in the expression of the transcriptional activator VjbR. The TCS BvrRS thereby controls the expression of Brucella’s T4SS VirB directly and indirectly. It appears that the co-regulation of Brucella genes encoding the synthesis or modification of cyclic-b-1,2-glucans with those encoding the T4SS secretion apparatus and/or secreted effectors could provide additional Brucella factors 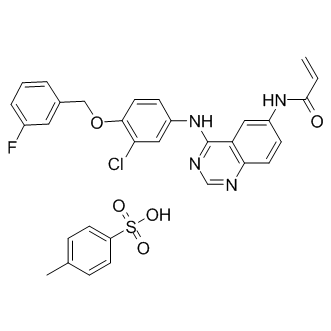 for cyclic-b-1,2-glucan’s mechanism in preventing phagosome maturation. External stimuli such as receptor activation by microbial toxins, pathogens and proteins induce the coalescence of the small DRMDs that are present in resting cells into larger ones. These clustered domains rapidly sequester proteins and lipids from detergent soluble membranes into DRMDs and serve as platforms for signal transduction, intracellular sorting, membrane transport, and possibly other functions. We reasoned that, as seen with other pathogens, pathogenic Brucella may rearrange proteins between DRMDs and detergent soluble membranes before it enters the host cell. In this study we analyzed the protein redistribution between DRMDs and non-DRMD membranes using a quantitative proteomic approach that compares protein profiles in DRMDs of monocytes exposed or not exposed to Brucella. We have developed a computational method to identify response networks in large biological networks based on expression data. This method and the corresponding computer program NetworkExpress is based on superimposing expression values upon the large network, identifying k-shortest paths between Nodakenin seed-nodes, scoring the sub-network spanned by the set of kshortest paths that are shorter than a pre-defined maximum weighted length l, and finding the best scored sub-network by optimization techniques. k-shortest paths refer to a set of paths between a start- and end-point in a network with shortest, second-shortest, third-shortest connections up to a given value k. Paths are weighted, thus connections between intermediate nodes along the path can be shorter or longer depending on the expression values and scoring function used. We have a variety of scoring functions available, from simple arithmetic or geometric means to different types of correlation functions for time-series correlations, optionally between same time-points or timeforward/backward. The best-scored sub-network refers to the response network of the system under the specific environmental condition measured by the corresponding expression experiment.
for cyclic-b-1,2-glucan’s mechanism in preventing phagosome maturation. External stimuli such as receptor activation by microbial toxins, pathogens and proteins induce the coalescence of the small DRMDs that are present in resting cells into larger ones. These clustered domains rapidly sequester proteins and lipids from detergent soluble membranes into DRMDs and serve as platforms for signal transduction, intracellular sorting, membrane transport, and possibly other functions. We reasoned that, as seen with other pathogens, pathogenic Brucella may rearrange proteins between DRMDs and detergent soluble membranes before it enters the host cell. In this study we analyzed the protein redistribution between DRMDs and non-DRMD membranes using a quantitative proteomic approach that compares protein profiles in DRMDs of monocytes exposed or not exposed to Brucella. We have developed a computational method to identify response networks in large biological networks based on expression data. This method and the corresponding computer program NetworkExpress is based on superimposing expression values upon the large network, identifying k-shortest paths between Nodakenin seed-nodes, scoring the sub-network spanned by the set of kshortest paths that are shorter than a pre-defined maximum weighted length l, and finding the best scored sub-network by optimization techniques. k-shortest paths refer to a set of paths between a start- and end-point in a network with shortest, second-shortest, third-shortest connections up to a given value k. Paths are weighted, thus connections between intermediate nodes along the path can be shorter or longer depending on the expression values and scoring function used. We have a variety of scoring functions available, from simple arithmetic or geometric means to different types of correlation functions for time-series correlations, optionally between same time-points or timeforward/backward. The best-scored sub-network refers to the response network of the system under the specific environmental condition measured by the corresponding expression experiment.