Collectively, these studies strongly suggest that TRPM7 may be an effective pharmacological target for stroke treatment; however, compounds that could potentially be used clinically against the channel have not been identified. In this study we have Temozolomide identified the 5-LOX inhibitors NDGA, AA861, and MK886 as potent blockers of TRPM7 channel activity. The compounds were also effective at inhibiting TRPM7 channel function, as application of these molecules prevented TRPM7-induced cell rounding as well as cell death caused by low extracellular divalent cations or several forms of apoptotic stimuli. NDGA, AA861, and MK886 were originally identified by their capacity to inhibit 5-LOX, however, several lines of evidence suggest that these compounds block TRPM7 channel currents directly and independent of their inhibitory effects on 5LOX enzymatic activity. Transfection of the dsiRNA targeting 5LOX failed to lower TRPM7 whole cell currents compared to cells transfected with the control dsiRNA, although transfection of dsiRNAs targeting the 5-LOX partially interfered with TRPM7mediated cell rounding. It has been reported that 5-LOX is involved in the regulation of cell adhesion, so the effects of the 5LOX dsiRNAs on TRPM7-induced cell rounding are likely due to direct knockdown of 5-LOX expression. In addition, we were unable to reverse AA861��s blockade of TRPM7 channel activity by perfusion of the 5-LOX product 5-HPETE or its downstream metabolites into the extracellular bath solution. Likewise, inclusion of either 5-HPETE, LTD4, and LTB4 into the internal pipette solution did not prevent the inhibition of TRPM7 channel activity by AA861. Finally, the other two 5-LOX inhibitors, 5,6-DAA and zileuton, were ineffective in blocking TRPM7 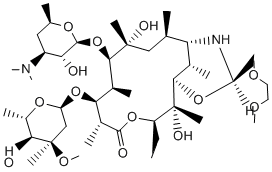 currents. Collectively, these results strongly Y-27632 dihydrochloride indicate that NDGA, AA861, and MK886 block TRPM7 channel currents independent of their actions on 5-LOX enzymatic activity. NDGA, AA861, and MK886 did not alter TRPM7 protein expression or its concentration on the cell surface, leaving it unclear how these compounds may be interfering with TRPM7 channel activity. NDGA is a lipophilic reducing agent that blocks catalysis by reducing the active site iron in 5-LOX, whereas AA861 competes with binding of arachadonic acid to the enzyme. The structurally unrelated indole-containing MK886 is also lipophilic, blocking 5-LOX activity by binding to FLAP, a membrane protein that facilitates 5-lipoxygenase enzymatic activity by enhancing the delivery of arachidonic acid to 5-LOX. Thus, the compounds may be blocking TRPM7 directly in the membrane or by interfering with binding of lipid to the channel. Since NDGA, AA861, and MK886 effectively block the endogenous TRPM7 current, a reevaluation of the results of experimental studies employing these compounds is warranted. Administration of 5-LOX inhibitors has been shown to reduce tissue damage in rodent models of cerebral ischemia and myocardial ischemia-reperfusion injury. However, no significant difference in the infarct size between control and 5-LOX knockout mice was observed using either a heart or brain model of ischemic injury. As knockdown of the TRPM7 channel reduces the pathogenesis of brain ischemia, it is tempting to speculate that 5-LOX inhibitors achieve a portion of their cellular protective effects by blocking the TRPM7 channel. Indeed, the 5-LOX inhibitors AA861 and NDGA were effective in reversing TRPM7-induced cell death when cells are cultured in low extracellular divalent cations. In addition, both knockdown of TRPM7 and application of AA861 were effective in reducing cell death caused by apoptotic stimuli.
currents. Collectively, these results strongly Y-27632 dihydrochloride indicate that NDGA, AA861, and MK886 block TRPM7 channel currents independent of their actions on 5-LOX enzymatic activity. NDGA, AA861, and MK886 did not alter TRPM7 protein expression or its concentration on the cell surface, leaving it unclear how these compounds may be interfering with TRPM7 channel activity. NDGA is a lipophilic reducing agent that blocks catalysis by reducing the active site iron in 5-LOX, whereas AA861 competes with binding of arachadonic acid to the enzyme. The structurally unrelated indole-containing MK886 is also lipophilic, blocking 5-LOX activity by binding to FLAP, a membrane protein that facilitates 5-lipoxygenase enzymatic activity by enhancing the delivery of arachidonic acid to 5-LOX. Thus, the compounds may be blocking TRPM7 directly in the membrane or by interfering with binding of lipid to the channel. Since NDGA, AA861, and MK886 effectively block the endogenous TRPM7 current, a reevaluation of the results of experimental studies employing these compounds is warranted. Administration of 5-LOX inhibitors has been shown to reduce tissue damage in rodent models of cerebral ischemia and myocardial ischemia-reperfusion injury. However, no significant difference in the infarct size between control and 5-LOX knockout mice was observed using either a heart or brain model of ischemic injury. As knockdown of the TRPM7 channel reduces the pathogenesis of brain ischemia, it is tempting to speculate that 5-LOX inhibitors achieve a portion of their cellular protective effects by blocking the TRPM7 channel. Indeed, the 5-LOX inhibitors AA861 and NDGA were effective in reversing TRPM7-induced cell death when cells are cultured in low extracellular divalent cations. In addition, both knockdown of TRPM7 and application of AA861 were effective in reducing cell death caused by apoptotic stimuli.