An insecticide targeting specifically the G119S AChE1 should efficiently reduce the LY2157299 frequency of the resistance allele, while the probability of OP-resistant mosquitoes developing a secondary resistance to the new insecticide is predicted to be quite low. To address the feasibility of this approach, compounds were screened for their capacity to inhibit more efficiently the G119S-substitutedAChE1 than the wild typeAChE1. Further biochemical analysis, bioassays on mosquito larvae from susceptible and resistant A. gambiae and C. pipiens strains sharing the same genetic background, as well as gene population modeling show that application of compounds with such properties is predicted to rapidly restore OP susceptibility in field populations. We show here that PTFs qualify as a new class of reversible and competitive AChE1 inhibitors, with preferential efficacy toward OP-insensitive AChE1 in vitro and OP-resistant C. pipiens or A. gambiae mosquito larvae in vivo. The fact that PTFs behave as reversible and competitive AChE1 inhibitors strongly suggests that they target the docking sites for acetylcholine, i.e. the peripheralor the activesites. Furthermore, PTFs preferentially inhibit all OP-insensitive mutants, whose substituted positions line the A-site and are thought to confer OP-insensitivity by steric hindrance. It is therefore unlikely that PTFs can freely enter the A-site of OP-insensitive enzymes and a more likely hypothesis is that PTFs bind on top of the A- or the P-site, thereby blocking access to the substrate to either site. This agrees with the absence of correlation between PTF efficacy and specific chemical groups at the R1,R 2 or R3 positions, suggesting that docking of the core PTF structure to its target might be stabilized through multiple interactions with neighboring residues. Availability of AChE1 three-dimensional structure, alone or in complex with PTFs, should help understand the structural basis of the interaction. PTFs proved to be efficient in vivo on C. pipiens and A. gambiae larvae and showed similar selectivity toward OP-resistant larvae, although with different RLD50 values. Behavioral or physiological differences might account for this difference; Anopheles gambiae larvae are surface filter-feeders while C. pipiens are deeper filterfeeders that regularly swim up to breathe. Previous work also demonstrated that the dominance of resistance in C. pipiens varies with environment, such as food, water quality and 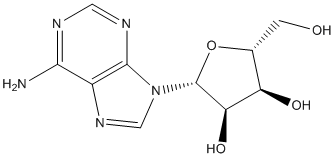 shape of the cups used in bioassays. All these parameters might impact on insecticide uptake or induced mortality. Since PTFs inhibit all types of OP-insensitive AChE1 in vitro, i.e. G119S-, F290V- or F331W-substituted, this strongly suggests that PTF molecules might exhibit a broad larvicidal spectrum toward most OP-resistant field populations. In conclusion, this study validates an innovative approach for resistance management of mosquito vectors, based on the development of molecules targeting preferentially enzymes already insensitive to MK-1775 955365-80-7 currently used insecticides. PTFs identified here behave as preferential inhibitors of AChE1 mutants insensitive to OP and CX and as preferential killers of OPresistant C. pipiens and A. gambiae larvae in bioassays. This approach should allow both efficient vector and resistance control management: The preferential killing of resistant mosquitoes mimics a situation of negative cross-resistance, in which frequency of resistance alleles decreases much faster than when insecticides acting on other targets are used. Furthermore, since wild type OP-susceptible alleles are, by construction, PTFresistant alleles, odds are against the selection of other resistance events.
shape of the cups used in bioassays. All these parameters might impact on insecticide uptake or induced mortality. Since PTFs inhibit all types of OP-insensitive AChE1 in vitro, i.e. G119S-, F290V- or F331W-substituted, this strongly suggests that PTF molecules might exhibit a broad larvicidal spectrum toward most OP-resistant field populations. In conclusion, this study validates an innovative approach for resistance management of mosquito vectors, based on the development of molecules targeting preferentially enzymes already insensitive to MK-1775 955365-80-7 currently used insecticides. PTFs identified here behave as preferential inhibitors of AChE1 mutants insensitive to OP and CX and as preferential killers of OPresistant C. pipiens and A. gambiae larvae in bioassays. This approach should allow both efficient vector and resistance control management: The preferential killing of resistant mosquitoes mimics a situation of negative cross-resistance, in which frequency of resistance alleles decreases much faster than when insecticides acting on other targets are used. Furthermore, since wild type OP-susceptible alleles are, by construction, PTFresistant alleles, odds are against the selection of other resistance events.