The mitochondrial theory of aging postulates that mutations in mitochondrial DNAaccumulate with age and result in impaired quality and activity of the mtDNA-encoded proteins. The theory is supported by the fact that mitochondrial function decreases with age, presumably due to accumulation of somatic mtDNA mutations. Mitochondrial dysfunction associated with accumulation of clonal expansions of deletion mutations are reported in nucleoside reverse transcriptase inhibitor treated 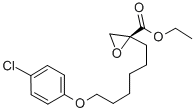 individualsand neurodegenerative disorders including Multiple Sclerosis, Alzheimer’s Disease and Parkinson’s Disease. Although mutated mtDNA are selectively removed during folliculogenesisas well as during maternal transmission, inheritable heteroplasmy causes inheritable mitochondrial disease like MELAS when such processes fail. The Polg mutator mouse expresses an error-prone mtDNA polymerase c and accumulates excessive mtDNA mutations in an age-dependent manner. The Polg mutator evidently demonstrates that mtDNA mutations can result in pathology and shortened lifespan. Although this model has been used to demonstrate the correlation between mtDNA mutations, mitochondrial dysfunction and premature aging, it is questionable to what extent this genetic mitochondrial mutator model represents the molecular mechanisms that underlie mitochondrial dysfunction during normal aging. For instance, the level of mtDNA substitution mutations in old individuals with a corresponding mitochondrial dysfunction does not produce a mitochondrial dysfunction when present in young mutator mice. Rather, mtDNA deletions were suggested to be responsible for age-mediated dysfunction. Accumulation of mtDNA deletions correlated with the phenotype and mutation frequency during normal aging. The deleterious functional effects of deletion mutations are demonstrated clinically in Kearns-Sayre Syndrome. However, genetic models have demonstrated that deletion mutations in up to 60% of the mtDNA molecules are tolerated without manifesting into phenotypic abnormalities. In view of the relatively high tolerance for mtDNA deletion mutations, there is an unexplained discrepancy between the observed mutation frequency during normal aging and the age-associated dysfunction. The functional impact of mtDNA mutations is hard to Atractylenolide-III predict because of the multiplicity of mtDNA molecules in the cell. mtDNA copy number is also subjected to variations and the heteroplasmic state implies that the likelihood for a mutation to manifest into dysfunctional protein depends on the mtDNA copy number, given that transcription occurs Sipeimine randomly among mtDNA molecules. In view of the redundancy of mtDNA to serve templates for downstream mitochondrial protein components, we reasoned that the best strategy to evaluate mtDNA mutagenesis would be to address the integrity of the mitochondrial RNA. In order for mtDNA mutations to result in functional impairment, the mutations must significantly modify the population of mtRNA molecules. To investigate the impact of mtDNA mutations with age, we developed an assay to determine mtRNA integrity with high resolution and used this technology to compare mtDNA mutagenesis and mtRNA error frequency in brains from young mice with those from old mice, which were associated with impaired mitochondrial function. Our results show that mtRNA error frequency can be used to validate mtDNA mutagenesis. The large variations in mutation frequency suggested that the penetrance might be site-specific.
individualsand neurodegenerative disorders including Multiple Sclerosis, Alzheimer’s Disease and Parkinson’s Disease. Although mutated mtDNA are selectively removed during folliculogenesisas well as during maternal transmission, inheritable heteroplasmy causes inheritable mitochondrial disease like MELAS when such processes fail. The Polg mutator mouse expresses an error-prone mtDNA polymerase c and accumulates excessive mtDNA mutations in an age-dependent manner. The Polg mutator evidently demonstrates that mtDNA mutations can result in pathology and shortened lifespan. Although this model has been used to demonstrate the correlation between mtDNA mutations, mitochondrial dysfunction and premature aging, it is questionable to what extent this genetic mitochondrial mutator model represents the molecular mechanisms that underlie mitochondrial dysfunction during normal aging. For instance, the level of mtDNA substitution mutations in old individuals with a corresponding mitochondrial dysfunction does not produce a mitochondrial dysfunction when present in young mutator mice. Rather, mtDNA deletions were suggested to be responsible for age-mediated dysfunction. Accumulation of mtDNA deletions correlated with the phenotype and mutation frequency during normal aging. The deleterious functional effects of deletion mutations are demonstrated clinically in Kearns-Sayre Syndrome. However, genetic models have demonstrated that deletion mutations in up to 60% of the mtDNA molecules are tolerated without manifesting into phenotypic abnormalities. In view of the relatively high tolerance for mtDNA deletion mutations, there is an unexplained discrepancy between the observed mutation frequency during normal aging and the age-associated dysfunction. The functional impact of mtDNA mutations is hard to Atractylenolide-III predict because of the multiplicity of mtDNA molecules in the cell. mtDNA copy number is also subjected to variations and the heteroplasmic state implies that the likelihood for a mutation to manifest into dysfunctional protein depends on the mtDNA copy number, given that transcription occurs Sipeimine randomly among mtDNA molecules. In view of the redundancy of mtDNA to serve templates for downstream mitochondrial protein components, we reasoned that the best strategy to evaluate mtDNA mutagenesis would be to address the integrity of the mitochondrial RNA. In order for mtDNA mutations to result in functional impairment, the mutations must significantly modify the population of mtRNA molecules. To investigate the impact of mtDNA mutations with age, we developed an assay to determine mtRNA integrity with high resolution and used this technology to compare mtDNA mutagenesis and mtRNA error frequency in brains from young mice with those from old mice, which were associated with impaired mitochondrial function. Our results show that mtRNA error frequency can be used to validate mtDNA mutagenesis. The large variations in mutation frequency suggested that the penetrance might be site-specific.